Electroplating is a process where a thin layer of metal is deposited onto the surface of an object, typically through an electric current. This process isn’t just for aesthetic purposes; it enhances the properties of the base metal, providing benefits like corrosion resistance, improved durability, or better electrical conductivity. But here’s the million-dollar question: why choose one metal over another for electroplating? Why is zinc better than tin for electroplating in many situations?
What is Electroplating?
In simple terms, electroplating involves coating an object with a thin layer of metal to improve its properties. The process typically uses an electrolyte solution containing the metal to be plated. Through the application of an electric current, the metal ions from the solution get deposited onto the object (which acts as the cathode in the process), while the anode generally contains the same metal or a related one.
This process is crucial in industries such as:
- Automotive manufacturing (plating of parts like bolts and chassis components).
- Electronics (coating connectors or contacts for better conductivity and protection).
- Jewelry (to give an elegant, shiny finish).
While the application of electroplating can vary widely, the most important factor remains the material used for plating. Zinc and tin are two of the most commonly used metals, each with distinct advantages and limitations. So, let’s look at zinc and tin in electroplating and see which one comes out on top.
Overview of Zinc and Tin in Electroplating
Zinc has been a popular choice for electroplating due to its unique properties. It provides excellent protection against corrosion, and its relatively low cost makes it a go-to for industries that require large-scale production. Zinc also creates a “sacrificial” barrier, meaning it corrodes before the base metal does, prolonging the life of the component beneath.
On the other hand, tin is also widely used in electroplating, especially in food-related applications (due to its non-toxic nature) and in electronics. Tin electroplating is often chosen for its non-corrosive properties in certain environments and its ease of application. However, despite these advantages, tin is not always the best option in every scenario. It lacks the same level of durability and corrosion resistance as zinc and can be less cost-effective, especially in heavy-duty applications.

Properties of Zinc and Tin in Electroplating
To understand why zinc is better than tin for electroplating, we need to examine the properties of each metal and how they behave during the electroplating process. From corrosion resistance to their interaction with other materials, these properties determine how well the metals will perform over time, especially in harsh environments.
Chemical Properties of Zinc
Zinc has several chemical properties that make it ideal for electroplating, especially when protection from corrosion is a priority. Here’s why:
- Corrosion Resistance: One of zinc’s most remarkable properties is its ability to prevent rust and corrosion. When zinc is plated onto a base metal like steel, it creates a protective layer that shields the metal from exposure to moisture and air. The secret is in galvanic protection: zinc corrodes first before the underlying metal, keeping the base material intact for a much longer period.
- Sacrificial Protection: The term “sacrificial” refers to how zinc acts as a “decoy” for corrosion. In environments where both zinc and the underlying metal are exposed to corrosive agents like water or air, zinc corrodes more readily, protecting the base metal. This is why zinc is often used in marine applications, construction, and the automotive industry—industries where corrosion protection is non-negotiable.
- Adhesion: Zinc adheres well to most base materials, allowing for consistent and durable electroplating layers. This results in a smooth and even surface finish, which is crucial for both aesthetics and functionality.
- Conductivity: While zinc is not as conductive as some other metals like copper or gold, it still provides adequate electrical conductivity for applications like electrical connectors or circuit boards, which makes it useful in electronics.
Chemical Properties of Tin
Tin, while not as universally beloved as zinc in electroplating, still has its own strengths. Here’s a look at tin’s properties in the electroplating world:
- Corrosion Resistance: Tin also resists corrosion but not quite to the extent that zinc does. Tin-plated surfaces are often used in environments where exposure to corrosion is minimal or in applications where corrosion protection is not the most critical factor. For example, tin is often used to coat food cans because it prevents rust, but it doesn’t have the same galvanic protection as zinc.
- Softness: One downside of tin is its relatively soft nature. This makes it more susceptible to wear and tear. In high-traffic or industrial applications, tin may wear down faster than zinc, leading to a shorter lifespan for the electroplated component. On the other hand, this softness can be beneficial for specific applications, such as in soldering, where the metal needs to be pliable.
- Non-Toxicity: Tin’s non-toxic properties make it a preferred choice in certain industries, particularly in food packaging and medical devices. This is one of the reasons why tin is frequently chosen for electroplating food cans, as it doesn’t pose a health risk when in contact with food.
- Adhesion: While tin does adhere well to base materials, it doesn’t always form the stronger bonds that zinc does. This can lead to less durability in certain applications.
Why Zinc is Better Than Tin for Electroplating
Now that we’ve covered the basic properties of both metals, it’s time to answer the core question: Why is zinc better than tin for electroplating? Let’s dive deeper into the specific advantages that zinc brings to the table.
Corrosion Resistance
When it comes to electroplating, corrosion resistance is often the make-or-break factor. Zinc is the king in this area. As mentioned earlier, zinc provides sacrificial protection, meaning it corrodes before the underlying material does. This unique ability allows zinc-plated components to withstand harsher environments, such as saltwater, acidic or basic conditions, and extreme weather. It’s no wonder zinc is commonly used in the automotive industry, where parts are exposed to rain, dirt, and road salt.
Tin, on the other hand, does offer some corrosion protection but doesn’t provide the same level of defense. Tin-plated materials are more vulnerable in highly corrosive environments. For example, if you compare a zinc-plated car part with a tin-plated one, the zinc-coated part will last significantly longer in the face of rust, especially in places prone to moisture and corrosion.
Cost-Effectiveness of Zinc Over Tin
Cost is always a factor in industrial decisions, and when you’re dealing with electroplating on a large scale, it’s no different. Zinc is a much more affordable option than tin, and that’s why it’s often preferred in industries where large volumes of electroplated items are needed.
Not only is zinc less expensive to source, but it also has a lower processing cost. This is especially true when considering that zinc is more readily available and can be plated faster and more efficiently than tin. For industries like construction, automotive manufacturing, and heavy machinery, where electroplating is needed in high quantities, zinc’s cost-effectiveness makes it the obvious choice.
On the other hand, tin’s higher cost and slightly more complicated plating process make it less ideal for mass production. While tin is preferred in certain applications (like food containers and some electrical components), it’s simply not as economically viable for large-scale electroplating jobs.
Durability and Longevity
When it comes to durability, zinc comes out on top. Zinc electroplating provides a thicker and stronger coating compared to tin, making it more resistant to wear and tear. Zinc-plated parts tend to last much longer under stressful conditions, which is why it’s the go-to for parts that endure high friction, moisture, and physical stress—such as automobile parts, construction materials, and marine hardware.
In contrast, tin’s softer nature means that it wears down more quickly, especially in situations where it is subjected to abrasion or heavy usage. This is one of the reasons why tin plating is often used in less demanding environments or in applications where the coating doesn’t need to be as durable.
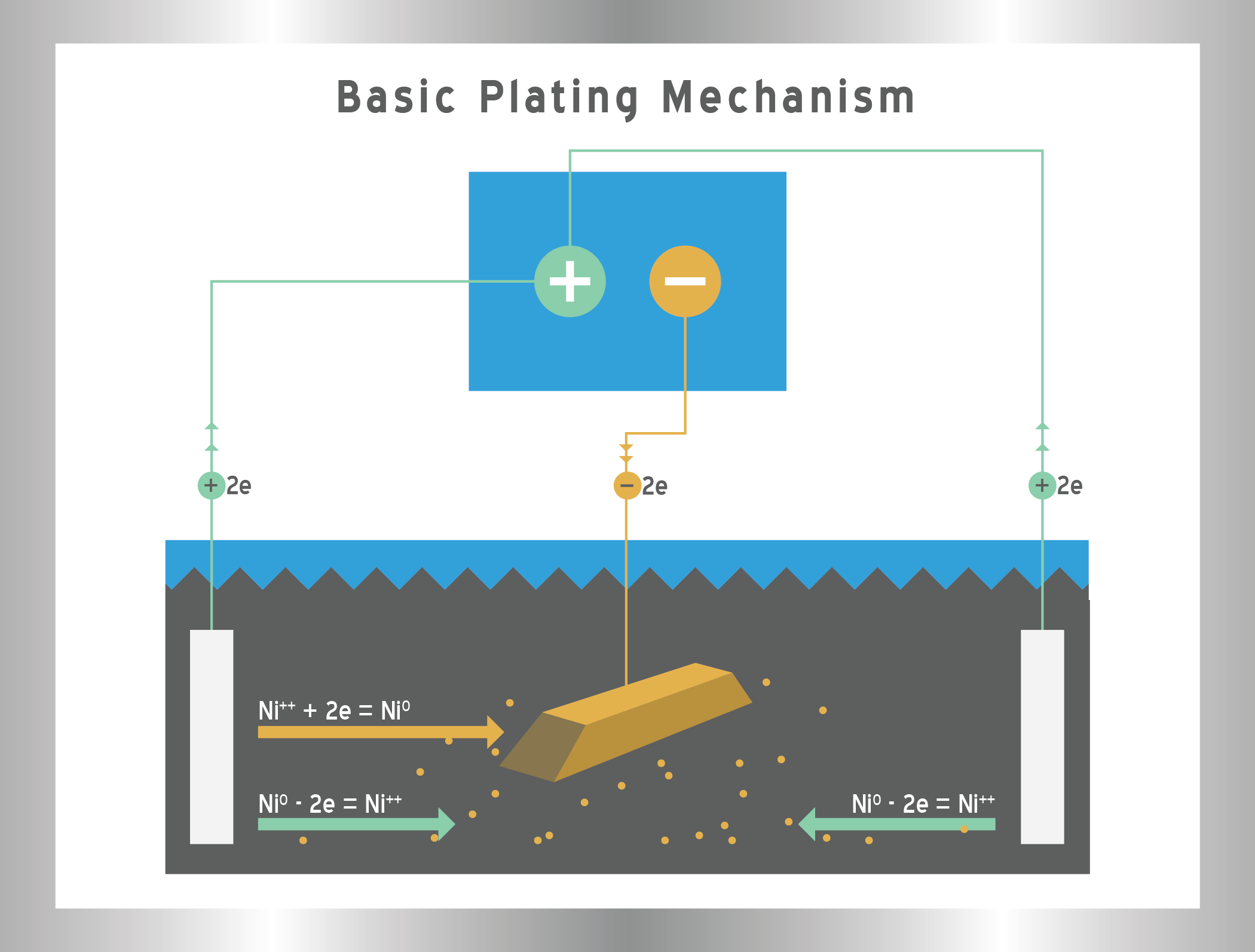
Ease of Plating and Adhesion
Another important consideration in electroplating is the ease of plating and the adhesion strength of the metal to the base material. Zinc has a significant advantage here over tin.
- Adhesion Strength: Zinc has excellent adhesion properties, which means it bonds effectively to most metals during the electroplating process. This is particularly important in industrial applications where the electroplated surface needs to be durable and not prone to peeling or flaking. Zinc’s ability to adhere strongly ensures that the plating lasts longer and performs better, even under physical stress.
- Ease of Plating: Zinc is easier to work with during the electroplating process. The plating bath for zinc is typically less complex, and it is easier to achieve consistent coating thickness. This is one reason why zinc electroplating is used extensively in industries where mass production is a factor, as it simplifies the manufacturing process.
On the flip side, tin plating is often trickier to apply. While tin does adhere well to surfaces, it doesn’t always form as strong a bond as zinc does. The tin-plating process also tends to require more careful attention to uniformity and thickness, and achieving the ideal coating can sometimes be more time-consuming and expensive.
For manufacturers who need to produce large quantities of plated parts efficiently and with minimal defects, zinc’s easier plating process is a big win.
Environmental Considerations
In today’s world, environmental impact is becoming an increasingly important consideration for industries worldwide. So, let’s talk about the environmental benefits of zinc compared to tin in electroplating.
- Zinc: Zinc has been a frontrunner in terms of sustainability. The electroplating process itself is relatively low in environmental impact, especially when compared to other metal coatings like chrome or nickel. Additionally, zinc plating can often be done without using toxic chemicals, which is a significant advantage in eco-conscious industries.
- Tin: Tin is also considered a relatively eco-friendly material, especially since it’s non-toxic. However, tin electroplating is typically associated with more complex processes and the use of more chemicals, which can increase the environmental footprint. Also, while tin plating is a safer alternative in terms of toxicity compared to some other materials, it doesn’t quite match the environmental advantages that zinc provides.
In short, if you’re considering the environmental impact of electroplating processes, zinc is often the better choice due to its lower chemical use and greater sustainability.
Applications of Zinc Electroplating
Now that we’ve explored why zinc is often the superior choice for electroplating, let’s dive into the real-world applications where zinc shines the brightest. From the automotive industry to electronics, zinc is everywhere, helping to improve durability and longevity in various components.
Automotive Industry
Zinc is heavily used in the automotive industry for electroplating parts that require enhanced protection from corrosion. Zinc is a natural choice for parts like bolts, nuts, and chassis components because it offers superior resistance to the elements, including road salts, moisture, and other corrosive substances. For instance, a zinc-plated car part exposed to rain or snow will experience far less rust than one that isn’t treated.
This corrosion protection ensures that cars last longer, which is especially important in regions with harsh weather conditions, like wintery climates where road salt is used extensively. Zinc’s ability to act as a sacrificial anode means that it will corrode first, leaving the underlying metal untouched and preserving the structural integrity of the car part.
Construction and Infrastructure
In the construction industry, materials like steel beams, fasteners, and hardware are commonly zinc-plated to prevent rust and corrosion. Zinc plating can increase the lifespan of these materials, reducing maintenance costs and improving the overall durability of infrastructure.
For example, zinc-plated steel is commonly used in bridges, buildings, and roofs, where exposure to rain, humidity, and even saltwater in coastal areas is a concern. The additional protective layer provided by zinc ensures that these essential structures remain intact over time.
Electronics and Electrical Components
Zinc is also used extensively in the electronics and electrical components industry. Zinc-plated electrical connectors are found in devices like phones, computers, and televisions, where reliable conductivity and corrosion resistance are essential.
While tin is also used in electronics, especially for soldering due to its non-toxicity, zinc electroplating is chosen when the electrical contacts or connectors need to resist corrosion while maintaining adequate conductivity. Zinc-plated connectors are more durable in environments where exposure to humidity and other corrosive agents could damage the components.
Other Industries (Marine, Aerospace, etc.)
Zinc’s corrosion resistance makes it an obvious choice for industries like marine and aerospace, where parts are subjected to extreme conditions. In marine environments, components like boat propellers, anchors, and ship hulls often get zinc electroplating to protect them from saltwater corrosion.
In aerospace, zinc electroplating is used for parts that need to resist the harsh conditions found at high altitudes, such as aircraft landing gear or aircraft fasteners. Zinc offers an additional layer of protection against corrosion while also helping to extend the life of these critical components.

Comparison of Zinc vs Tin Electroplating in Various Scenarios
We’ve already established that zinc often outperforms tin in many electroplating applications. However, it’s important to recognize that there are certain situations where tin plating may still be the better choice. Let’s take a closer look at different scenarios to understand when each metal might be preferred.
When to Choose Zinc Over Tin
In most industrial and commercial electroplating applications, zinc is the go-to material, and here’s why:
- High Corrosion Resistance Needed: If the plated component will be exposed to harsh environmental conditions, such as in the automotive, construction, or marine industries, zinc should be the clear choice. Its sacrificial protection (where zinc corrodes before the base metal) is especially important in these scenarios.
- Cost Constraints: When working on a large-scale manufacturing project, especially in sectors like automotive or heavy machinery, zinc is typically the more cost-effective choice. Its lower material cost and faster plating process make it an appealing option for industries that require plating in high volumes.
- Durability in Harsh Environments: If the component will face heavy wear and tear or mechanical stress (e.g., steel parts, construction fasteners, or hardware), zinc is generally the better choice for its superior durability. It resists corrosion and wear much better than tin, leading to longer-lasting products.
When Tin Might Still Be Preferable
While zinc is better suited for most industrial applications, tin does have its place in the world of electroplating, especially in niche scenarios:
- Food and Beverage Industry: Tin is often the preferred metal for plating food cans and other food-related containers. Its non-toxic properties make it ideal for these applications, as it doesn’t pose any health risks. For example, tin is used in the electroplating of steel cans that store food, ensuring they’re safe for consumption while also preventing rusting and contamination.
- Soldering and Electronics: Tin electroplating is a popular choice in the electronics industry for soldering purposes. Tin-lead alloys have traditionally been used for their excellent soldering capabilities, though lead-free alternatives have become more common. Tin is preferred here because it flows easily during the soldering process and bonds well to various substrates.
- Aesthetic or Decorative Coatings: Tin can also be used for decorative purposes, especially when a shiny, bright finish is required. While zinc can also be used for aesthetic purposes, tin plating can sometimes offer a more aesthetically pleasing and uniform finish, especially in applications like jewelry, silverware, or certain electronics.
- Non-Corrosive, Low-Stress Applications: For low-stress applications, where parts aren’t exposed to harsh environments or wear, tin might be selected due to its low cost and adequate performance. Think of household electrical connectors or light-duty mechanical parts, where durability and corrosion resistance aren’t as critical.
Challenges and Considerations in Zinc Electroplating
While zinc electroplating offers a plethora of benefits, it’s not without its challenges.
Zinc Plating Process Challenges
Though zinc is relatively easy to plate, there are still some challenges that manufacturers may face during the process:
- Poor Adhesion: Occasionally, zinc may not adhere properly to the base metal, leading to flaking or peeling. To ensure better adhesion, surface preparation (such as cleaning, acid pickling, and abrasive blasting) is essential. Without proper surface treatment, the electroplated layer may fail to form a strong bond with the underlying material.
- Plating Defects: Just like with any plating process, defects such as uneven coatings, pitting, and dull finishes can occur. These defects are often caused by issues with the electroplating bath (e.g., imbalances in temperature or chemical composition). Monitoring and adjusting the bath conditions is crucial to achieving consistent plating results.
- Zinc Plating Thickness and Uniformity: Achieving the right plating thickness can be challenging. Too thin, and the coating may not provide sufficient protection; too thick, and it could interfere with the part’s functionality or lead to increased costs. It’s important to adjust the process to achieve the optimal thickness and uniformity for each application.
Zinc Plating vs Hot-Dip Galvanizing
One common misconception is that zinc electroplating and hot-dip galvanizing are the same thing. However, there are distinct differences between these two methods:
- Zinc Electroplating: Involves a thin coating of zinc applied through an electrolytic process. It’s ideal for components that require a fine, uniform coating with high corrosion resistance but are not exposed to extremely harsh conditions.
- Hot-Dip Galvanizing: Involves dipping parts into molten zinc, which creates a thicker and more durable coating. This process is typically used for larger components like steel beams, fencing, and construction materials that will face heavy exposure to weather and corrosion.
While zinc electroplating is perfect for precision parts and small components, hot-dip galvanizing is better suited for heavy-duty applications requiring thicker coatings.
Is Zinc Always the Better Choice for Electroplating?
While tin still holds its place in certain niche applications like food packaging and soldering, zinc is the clear winner in most electroplating scenarios. From its exceptional corrosion resistance to its cost-effectiveness and durability, zinc provides far more benefits for industries that rely on electroplating. Whether you’re in automotive, construction, or electronics, choosing zinc for electroplating ensures long-lasting protection, efficiency, and cost savings.
However, there will always be cases where tin’s specific properties—like its non-toxicity and aesthetic appeal—make it the better choice. The key takeaway here is that while zinc is better than tin for electroplating in the majority of cases, each metal’s properties should be carefully evaluated to determine the best fit for the job.
As the electroplating industry evolves, we may see new innovations that change the game, but for now, zinc reigns supreme.