When you think about shiny jewelry, durable car parts, or even high-tech electronics, there’s a good chance that electroplating played a role in giving them their distinctive finish. But what exactly is electroplating, and why do we rely on it to make products more durable, beautiful, and functional?
Electroplating is a fascinating process where a thin layer of metal is deposited onto the surface of an object using electrical current. This isn’t just about making things shiny (though, let’s be honest, that’s a big part of it!). The process improves a material’s properties, such as corrosion resistance, durability, electrical conductivity, and even aesthetics.
However, the real magic happens with the metal that’s used in electroplating. Choosing the right metal is crucial. From gold plating for luxurious jewelry to nickel plating for hard-wearing industrial parts, each metal has its unique properties that make it suitable for specific applications.
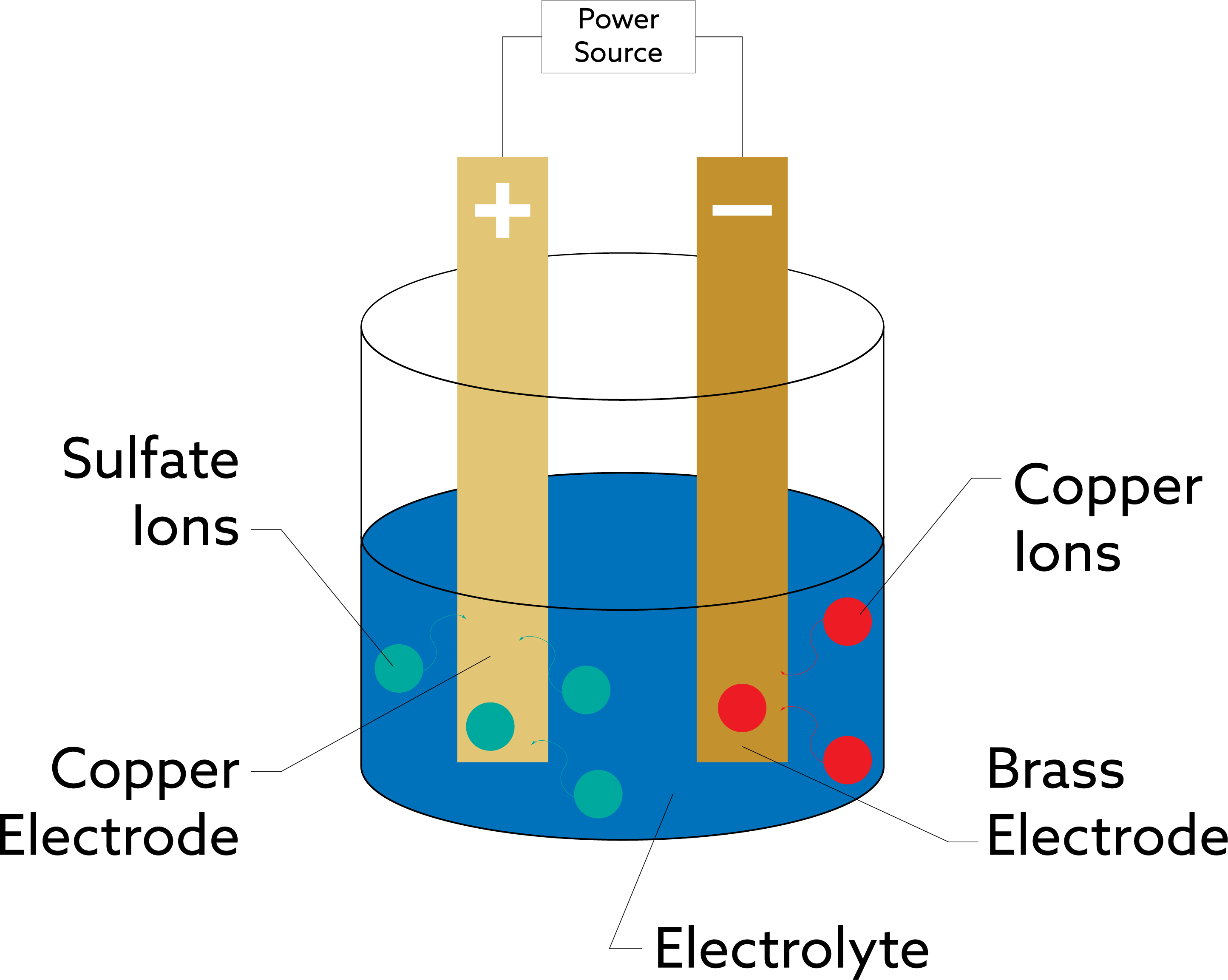
What is Electroplating and How Does It Work?
What Is Electroplating?
Electroplating is like giving a coat of armor to a surface, but not just any armor—a shiny, functional, and durable one. In simple terms, electroplating is the process of using electrical current to bond a thin layer of metal onto a surface. The object you want to plate (called the substrate) is placed in a bath containing a solution with dissolved metal ions. The electrical current causes the metal ions to migrate to the object’s surface, where they are reduced and form a solid metal coating.
Here’s a quick rundown of how electroplating works:
- Preparation: The substrate (usually metal) is cleaned thoroughly to remove dirt, oil, or oxidation.
- Electrolyte Bath: The object is submerged in an electrolyte solution containing the metal to be plated. For example, a gold electroplating bath will contain gold salts dissolved in a liquid solution.
- Electric Current: A current is passed through the bath, which causes the metal ions to be deposited onto the substrate, forming a smooth and durable coating.
The process sounds simple enough, but there’s a lot of science involved in making sure the plating is done correctly. Factors like temperature, voltage, and solution composition all affect the quality of the final result. In fact, achieving the right thickness and uniformity of the metal layer can take a bit of practice and precision.
Why is Electroplating Used?
Electroplating is used across many industries, from jewelry making to electronics manufacturing, because it enhances the properties of the material being plated. Here are some of the top reasons why electroplating is used:
- Improved Durability: Plating with certain metals like nickel or chrome can greatly improve the lifespan of an object by making it resistant to wear and tear.
- Corrosion Resistance: Metals like gold and silver are incredibly resistant to rust and oxidation, making them ideal for products exposed to the elements.
- Aesthetic Appeal: Electroplating adds a shiny, polished finish to items like jewelry, making them more visually appealing.
- Conductivity: Metals like gold and silver are excellent conductors of electricity, which is why they’re often used in electronics.
Whether you’re looking to protect your belongings from rust or create an eye-catching product, electroplating helps achieve these goals efficiently and effectively.

Commonly Used Metals in Electroplating
When it comes to electroplating, the type of metal you choose can significantly impact the outcome of the process. Each metal has its own set of unique properties that make it ideal for specific applications.
What Are the Most Common Metals Used for Electroplating?
1. Gold Electroplating
Why is Gold Used for Electroplating? Gold has been used for thousands of years for its beauty and durability, but in the world of electroplating, its main advantage lies in its corrosion resistance and excellent electrical conductivity. A thin layer of gold plating can significantly improve the longevity of items that are exposed to harsh environments, like electronics.
Applications:
- Electronics: Gold is commonly used to plate electrical connectors, circuit boards, and other components that require a highly conductive surface.
- Jewelry: Because of its rich yellow color and luster, gold is often used to plate cheaper metals or to create decorative finishes on jewelry.
- Aerospace and Military: In specialized applications, gold plating is used for components that must withstand extreme conditions while maintaining reliable electrical connections.
Benefits:
- Excellent conductivity: This is why gold plating is so popular in the electronics industry.
- Aesthetic appeal: Gold-plated items have a premium look, making them highly desirable in jewelry and luxury items.
2. Silver Electroplating
Why is Silver Used for Electroplating? Silver is another metal widely used in electroplating because it offers great electrical conductivity and a shiny, reflective finish. Though not as expensive as gold, silver offers a similar aesthetic appeal with a slightly different look and feel. It’s often used when gold plating is too costly, but the customer still wants a high-quality finish.
Applications:
- Jewelry and Silverware: Silver plating is popular for creating decorative finishes on jewelry, flatware, and decorative items.
- Electrical Contacts: Silver’s high conductivity makes it ideal for electroplating connectors, switches, and circuit boards.
Benefits:
- Excellent conductivity: Much like gold, silver is highly conductive, making it ideal for electrical applications.
- Cost-effective: Silver is more affordable than gold, making it a good alternative for those who want similar performance without the high price.
3. Nickel Electroplating
Why is Nickel Used for Electroplating? Nickel is one of the most commonly used metals in electroplating because of its durability and corrosion resistance. Unlike gold or silver, nickel is often used for industrial applications where strength and protection are paramount. Nickel plating is hard, making it an ideal choice for coatings that will be exposed to wear and tear.
Applications:
- Automotive parts: Nickel plating is commonly used on car parts like wheels, exhaust systems, and engine components to protect them from corrosion.
- Industrial Machinery: Nickel is used to coat machinery parts that experience heavy use, such as pumps, valves, and shafts.
- Plumbing Fixtures: Faucets, showerheads, and other plumbing items benefit from nickel’s corrosion-resistant properties.
Benefits:
- Hardness: Nickel provides a tough, hard surface that resists scratches, wear, and corrosion.
- Corrosion resistance: Nickel-coated objects can withstand moisture and chemicals, making them perfect for outdoor and industrial use.
4. Chrome Electroplating
Why is Chrome Used for Electroplating? Chrome is another popular metal for electroplating, particularly for its high reflectivity, hardness, and corrosion resistance. Chrome plating is often associated with sleek, shiny finishes that are both durable and visually striking. Chrome’s ability to resist tarnishing and corrosion makes it a favorite in many industries.
Applications:
- Automotive parts: Chrome is used extensively for bumpers, grills, and trim on cars, adding both aesthetic value and durability.
- Household Products: Kitchen faucets, bathroom fixtures, and hardware often feature chrome plating to enhance appearance and resist corrosion.
- Heavy machinery: Chrome plating is used on industrial equipment and machinery that is subjected to friction, such as pistons, cylinders, and shafts.
Benefits:
- Shiny finish: Chrome plating creates a smooth, shiny surface that is highly reflective, adding a polished, attractive look to any product.
- Corrosion resistance: Chrome plating is highly resistant to rust and corrosion, making it ideal for both indoor and outdoor products.
5. Copper Electroplating
Why is Copper Used for Electroplating? Copper is one of the most widely used metals for electroplating, particularly due to its excellent electrical conductivity and relatively low cost. It is often used as an intermediate layer in electroplating before applying other metals like nickel or gold. Copper’s smooth finish also makes it a popular choice for items that require a fine, smooth surface.
Applications:
- Printed Circuit Boards (PCBs): Copper is often electroplated onto PCBs to form the conductive pathways that allow electronic devices to function.
- Wiring: Copper is widely used in the creation of electrical wires and cables due to its low resistance and high conductivity.
Benefits:
- Conductivity: Copper is an excellent conductor of electricity, making it ideal for electronic applications.
- Cost-effective: Copper is more affordable than gold and silver, making it a go-to choice in many industries.
6. Zinc Electroplating
Why is Zinc Used for Electroplating? Zinc is a very affordable and effective metal for electroplating, particularly for items that require basic corrosion protection. Zinc plating is often used on steel to prevent rusting and improve the longevity of the material.
Applications:
- Automotive parts: Zinc is used to coat steel parts like nuts, bolts, and screws to protect them from rust and corrosion.
- Construction materials: Zinc is applied to steel in the construction industry, where it helps prevent rust in exposed areas.
Benefits:
- Corrosion protection: Zinc creates a barrier against rust, making it ideal for outdoor and industrial products.
- Low cost: Zinc is one of the most affordable metals used in electroplating, making it ideal for mass production.
Common Metals Used for Electroplating
| Metal | Primary Benefits | Common Applications | Notable Characteristics |
|---|---|---|---|
| Gold | Excellent conductivity, corrosion resistance | Electronics, jewelry, aerospace, military | Expensive, high aesthetic appeal, tarnish-resistant |
| Silver | High conductivity, shiny finish | Jewelry, flatware, electrical components | Tarnishes over time, less costly than gold |
| Nickel | Hard, durable, corrosion-resistant | Automotive parts, machinery, plumbing | Tough, resistant to wear and corrosion |
| Chrome | Shiny, corrosion-resistant, durable | Automotive parts, household fixtures, heavy machinery | High reflectivity, scratch-resistant |
| Copper | Excellent conductivity, cost-effective | Electronics, PCBs, wiring | Smooth finish, widely used in electronics |
| Zinc | Affordable, corrosion-resistant | Automotive parts, construction materials, fasteners | Good corrosion protection, economical |
That’s a detailed look at some of the most common metals used for electroplating. Each metal brings something different to the table, whether it’s the shiny luster of gold or the strength and corrosion resistance of nickel. But how do you choose the right one for your project?
How to Choose the Right Metal for Electroplating?
Choosing the right metal for electroplating isn’t just about picking the shiniest option—it’s about understanding the specific needs of your project. Whether you’re working on electronics, automotive parts, jewelry, or industrial machinery, selecting the best metal can make a huge difference in performance, cost, and aesthetics.
What Factors Should You Consider When Choosing a Metal for Electroplating?
Here are the main factors to keep in mind when selecting a metal for electroplating:
1. Durability and Resistance to Wear and Tear
If you’re working on items that will experience a lot of friction or use, durability is a top priority. Metals like nickel and chrome are well-known for their ability to withstand abrasion and harsh environments. These metals form a tough layer that resists scratches, dents, and general wear and tear.
Example: For industrial machinery, nickel plating is often chosen because it creates a hard surface that resists damage during operation, ensuring the equipment lasts longer.
2. Aesthetic Appearance (Color, Shine, Texture)
If your project is more about appearance (think jewelry or decorative items), then the aesthetic qualities of the metal become more important. Gold, silver, and chrome are all highly reflective and provide a luxurious, shiny finish that’s perfect for high-end products. The color of the metal also plays a role—gold provides a rich yellow hue, silver gives off a cool, polished look, and chrome offers a mirrored, silvery shine.
Example: For jewelry, gold electroplating is often used to give base metals like copper or brass a high-end look, adding both beauty and value.
3. Electrical Conductivity
For applications involving electronics or electrical components, the electrical conductivity of the plating metal is a critical factor. Gold and silver are highly conductive and are therefore ideal for plating connectors, circuit boards, and other electrical contacts. While copper is also a great conductor, it is often used as an intermediate layer before other metals are applied.
Example: Gold plating is commonly used for electronics like smartphone connectors or microchips, where conductivity and reliable performance are essential.
4. Corrosion Resistance
For items that will be exposed to moisture, chemicals, or extreme weather conditions, corrosion resistance should be a top priority. Metals like gold, chrome, and nickel are highly resistant to rust and corrosion, which makes them perfect for outdoor products, automotive parts, and marine applications. If the item will be exposed to harsh conditions regularly, a corrosion-resistant metal can prolong its life.
Example: Chrome plating is often used in the automotive industry for parts like car bumpers and grills, where resistance to rust is critical due to constant exposure to moisture, salt, and dirt.
5. Cost Considerations
Not all metals are created equal when it comes to cost. While gold and silver are valuable metals, they are also expensive, so they may not be suitable for every project. Nickel, copper, and zinc, on the other hand, are more affordable and are often used for industrial applications where cost-effectiveness is key. Zinc plating is particularly popular for fasteners and automotive parts, providing good protection at a lower price point.
Example: For mass-produced products like nuts, bolts, and screws, zinc plating is often chosen because it provides excellent corrosion resistance without breaking the bank.
What Are the Environmental Considerations of Different Electroplating Metals?
With increasing focus on sustainability and environmental responsibility, it’s important to consider the environmental impact of the metals used in electroplating. Some metals, such as cadmium and lead, have fallen out of favor due to their toxic properties, and environmental regulations have become stricter in recent years. For this reason, many industries have turned to eco-friendly alternatives or sought ways to reduce the environmental impact of their electroplating processes.
Eco-friendly Alternatives
- Nickel and copper are more commonly used today because they are relatively safe and environmentally friendly compared to metals like cadmium or lead.
- Zinc is another environmentally friendly option due to its natural corrosion resistance, and it’s widely used in the automotive and construction industries.
Example: Some companies now use nickel-phosphorus or nickel-boron electroplating methods as eco-friendly alternatives to traditional chrome plating, which often uses harmful chemicals like hexavalent chromium.
Waste Management and Disposal
- Proper disposal of electroplating waste is crucial, especially since many metals used in electroplating can be hazardous. To minimize environmental harm, wastewater from the electroplating process is often treated to remove harmful chemicals before being released.
- Regulatory standards in various regions require that businesses follow strict disposal guidelines to reduce the impact of the plating process on the environment.
Summary of Key Factors to Consider
| Factor | Considerations | Best Metals for Electroplating |
|---|---|---|
| Durability | Resistance to wear, abrasion, and friction. | Nickel, Chrome |
| Aesthetic Appearance | Desired color, shine, and smooth texture. | Gold, Silver, Chrome |
| Electrical Conductivity | Need for efficient electrical flow. | Gold, Silver, Copper |
| Corrosion Resistance | Resistance to rust and corrosion, especially in harsh environments. | Gold, Nickel, Chrome |
| Cost Considerations | Affordability for large-scale production or budget-conscious projects. | Zinc, Copper, Nickel |
| Environmental Impact | Eco-friendly plating processes and sustainable metals. | Nickel, Copper, Zinc |
That wraps up the key factors to consider when choosing the right metal for electroplating. It’s all about matching the right material to the specific needs of your project. Whether you need something durable, beautiful, or affordable, there’s a metal that fits the bill.

What Are the Benefits of Electroplating with Different Metals?
Electroplating offers a wide range of benefits depending on the metal used. Each metal has distinct characteristics that make it suitable for various applications, from enhancing the appearance of jewelry to improving the durability of industrial parts.
Gold Electroplating Benefits
Gold is synonymous with luxury and durability, and when it comes to electroplating, it’s no different. Here are the top benefits of using gold in the electroplating process:
- Excellent Electrical Conductivity: One of the primary reasons gold is used in electroplating is its exceptional ability to conduct electricity. This makes it the preferred choice for electrical connectors, microchips, and other components in high-end electronics. The gold-plated contacts ensure a stable connection and reliable performance.
- Corrosion Resistance: Gold is naturally resistant to corrosion and tarnishing. This makes it ideal for items that need to stand up to moisture or harsh environments. For example, gold-plated jewelry doesn’t tarnish easily, retaining its luster over time.
- Aesthetic Appeal: Gold plating adds a brilliant, rich color to products, making them more attractive. Whether it’s for jewelry, luxury watches, or decorative items, gold electroplating provides a high-end finish that is hard to beat.
- Durability: While gold itself is a soft metal, a thin layer of gold plating can provide a strong, protective coating for more delicate base metals. It’s an excellent way to combine the beauty of gold with the strength of other materials.
Example: High-end electronics, like smartphone connectors and computing components, often use gold plating because of its combination of conductivity and corrosion resistance.
Silver Electroplating Benefits
Silver plating offers a range of advantages, especially for applications that require both aesthetic appeal and performance. Here are the main benefits of electroplating with silver:
- Superb Electrical Conductivity: Silver is the best conductor of electricity among all metals, which makes it ideal for applications where electrical performance is critical, such as in printed circuit boards (PCBs), connectors, and switches.
- Shiny, Lustrous Finish: Silver provides a sleek, shiny appearance that’s highly desired in jewelry, flatware, and decorative items. Silver-plated products have an elegant, high-quality look, making them very attractive to consumers.
- Tarnish Resistance (with Proper Care): While silver can tarnish over time, silver electroplating often incorporates anti-tarnish coatings to protect the finish. This makes it more resistant to oxidation than plain silver.
- Cost-Effective Alternative to Gold: Silver plating provides a luxurious appearance similar to gold plating, but at a fraction of the cost. This makes it a popular choice for products that need to look high-end without the high price tag.
Example: Silver-plated jewelry is a popular choice for those looking for an affordable alternative to gold, offering both beauty and performance without breaking the bank.
Nickel Electroplating Benefits
Nickel is a workhorse when it comes to electroplating. Known for its hardness and corrosion resistance, nickel plating is used across a wide range of industries. Here’s why nickel is so beneficial:
- Enhanced Durability: Nickel plating provides a hard and wear-resistant coating that can withstand rough handling, friction, and impact. This is especially valuable in industrial applications, such as machinery and automotive parts.
- Corrosion Resistance: Nickel forms a protective layer that shields the underlying material from moisture, chemicals, and rust. This makes it ideal for outdoor applications where parts need to endure harsh conditions, such as plumbing fixtures or engine components.
- Improved Surface Finish: Nickel plating smooths out the surface of the base material, making it look sleek and uniform. It also offers a slightly shiny finish, which is often used as a base layer before applying a layer of chrome or gold for added aesthetic appeal.
- Versatility: Nickel is used for many applications across various industries, from electronics and aerospace to home goods and tools. It’s one of the most versatile metals used in electroplating.
Example: Nickel-plated automotive parts such as grills, wheels, and exhaust pipes are commonly found because of nickel’s ability to resist corrosion while maintaining strength and performance.
Chrome Electroplating Benefits
Chrome plating is a popular choice for applications where shiny finishes and corrosion protection are important. Here’s why chrome is a go-to metal for many electroplating projects:
- Aesthetic Appeal and Reflective Finish: Chrome is known for its high reflectivity and brilliant, mirror-like finish. Whether on automotive bumpers, furniture, or hardware, chrome plating enhances the appearance and adds a sleek, modern look.
- Durability and Hardness: Chrome plating creates a hard, scratch-resistant surface that is perfect for applications where wear and tear are common. Chrome-plated car parts and machinery often last longer because of this durable coating.
- Corrosion Resistance: Chrome is highly resistant to rust and corrosion. This makes it ideal for outdoor products, automotive parts, and kitchen appliances that need to withstand exposure to moisture and chemicals.
- Ease of Maintenance: Chrome-plated surfaces are easy to clean and maintain. A quick wipe-down keeps products looking brand new, making it a low-maintenance choice for many industries.
Example: Chrome-plated bathroom fixtures, such as faucets and showerheads, are highly popular because they not only look good but also resist rust and corrosion.
Copper Electroplating Benefits
Copper electroplating is most commonly used in electronics and wiring because of its excellent electrical conductivity and relatively low cost. Let’s explore the benefits of copper plating:
- Excellent Electrical Conductivity: Copper is the second-best conductor of electricity after silver, making it ideal for electrical applications. Copper electroplating is used in wires, circuit boards, and electrical connectors where high conductivity is essential.
- Cost-Effective: Copper is much more affordable than gold or silver, so it’s often used as a base metal or for intermediate layers before applying a more expensive plating like gold or nickel.
- Smooth Surface Finish: Copper provides a smooth, even coating that enhances the quality of the final product, which is especially important for electronics that require fine, uniform surfaces.
- Improved Adhesion: Copper plating is often used as a bonding layer between the base material and other plating materials, helping to improve the adhesion of subsequent coatings, such as nickel or gold.
Example: Printed circuit boards (PCBs) often undergo copper electroplating to ensure reliable performance in electronics and high-tech gadgets.
Zinc Electroplating Benefits
Zinc is an affordable, effective choice for electroplating, especially for items that need protection from corrosion without breaking the bank. Here are the key benefits of zinc plating:
- Corrosion Protection: Zinc is primarily used for its ability to resist rust. Zinc plating creates a protective layer that shields steel and other materials from moisture and oxygen, preventing rust formation. This makes it ideal for automotive parts, fasteners, and construction materials.
- Cost-Effective Solution: Zinc is one of the most affordable plating metals, which is why it’s commonly used in mass production. It provides a balance of performance and cost-efficiency.
- Durability: While not as tough as nickel or chrome, zinc still offers good durability for less demanding applications. It helps increase the lifespan of parts that are exposed to the elements, reducing the need for frequent replacements.
Example: Zinc-plated screws and bolts are commonly used in construction and manufacturing due to their ability to resist corrosion at an affordable price.
That’s a deep dive into the benefits of electroplating with different metals. As you can see, each metal has distinct advantages that make it suitable for specific applications. Whether you’re looking for a cost-effective solution or a high-performance coating, electroplating with the right metal can make all the difference.