Electroplating is a fascinating process that involves applying a thin layer of metal onto a surface through an electrochemical reaction. Whether you’re trying to enhance the appearance of jewelry, protect machinery parts from corrosion, or create intricate electronic components, electroplating plays a crucial role in modern manufacturing and product design.
But if you’ve been involved in electroplating or are looking into it, you’ve likely encountered the question: Is high current good for electroplating? This is not a straightforward yes or no answer. In fact, it involves understanding a variety of factors—such as the type of metal you’re plating, the quality of the finish you need, and the exact plating process you are following.

What is Electroplating?
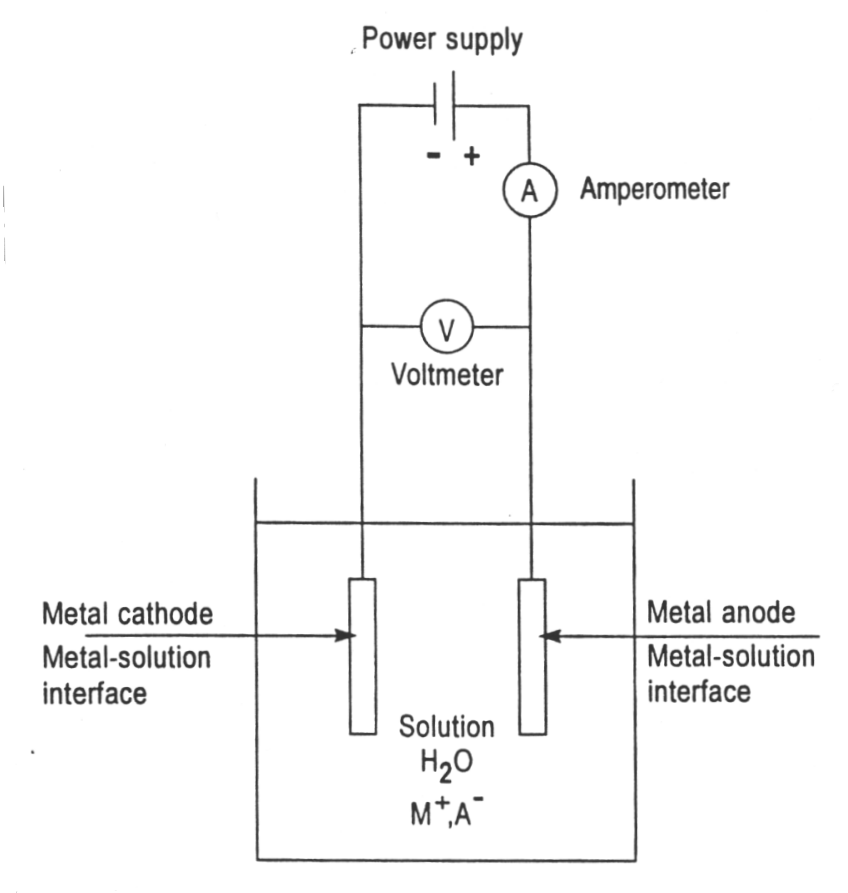
Before we can discuss the impact of high current on electroplating, it’s essential to first understand what electroplating actually is. At its core, electroplating is a process that uses electricity to reduce metal cations from a solution and deposit them as a thin layer onto a substrate (the material you’re plating). The result is a metal coating that can serve a wide variety of purposes, from providing corrosion resistance to creating a shiny, decorative finish.
The process works as follows:
- Electrolyte Solution: A solution containing metal salts, typically the metal you’re plating with, like gold, silver, or copper.
- Anode: A metal electrode, usually made of the metal you’re plating with, which will dissolve into the electrolyte.
- Cathode: The object or surface being plated, which is typically connected to the negative terminal of the power supply.
- Electric Current: This is applied to the anode and cathode to cause the metal ions in the electrolyte to migrate and plate the cathode.
The success of the electroplating process depends on several factors, such as the temperature of the electrolyte, the concentration of metal ions, and importantly, the current. It’s this electric current that drives the deposition of metal ions onto the cathode, so understanding how current affects the process is key to achieving the desired result.
How Does Current Affect the Electroplating Process?
Current plays a pivotal role in the electroplating process. Without it, the plating wouldn’t happen at all. But it’s not just about turning up the current to see what happens—there’s a careful balance that needs to be struck.
Here’s how current influences electroplating:
- Ion Transfer and Deposition: When current is applied, it causes the metal ions from the electrolyte solution to migrate toward the cathode, where they are reduced and deposited as solid metal. The amount of current used directly affects the rate of ion transfer, which influences how quickly metal gets deposited onto the substrate.
- Deposition Rate: Higher current can lead to a faster rate of deposition. However, while this might seem like a good thing if you’re looking to plate quickly, there’s more to the story. High current can result in thicker, faster plating, but it may also cause uneven deposits or introduce defects into the layer. For example, a too-thick plating may have poor adhesion or be rough around the edges. So, faster isn’t always better.
- Current Density: Current density refers to the amount of current flowing through a given surface area. Higher current densities can result in uneven deposits, especially in areas where the surface is complex or has sharp edges. Low current densities, on the other hand, typically produce smoother and more uniform deposits.
- Electrochemical Reactions: Higher current can also cause side reactions in the electrolyte, such as the production of gas bubbles (like hydrogen). These side reactions can interfere with the quality of the plating and the efficiency of the process.
In short, current is essential to electroplating, but too much or too little can cause problems. So, let’s move on and explore what actually happens when we increase the current.

What Happens When You Use High Current for Electroplating?
Now that we’ve established how current works in electroplating, let’s dive into the specific effects of using high current in the process. You might assume that more current equals better results, but as with most things in life, too much of a good thing can lead to complications. Here’s what happens when high current is applied during electroplating:
1. Faster Deposition of Metal
When you apply higher current, the rate of metal deposition increases. Essentially, more metal ions are being reduced at a faster rate and deposited onto the substrate. This can be advantageous in situations where speed is critical, such as in industrial settings where high-volume plating is necessary.
However, while it’s tempting to think that higher current equals thicker, faster plating, this isn’t always ideal. A rapid deposition can result in uneven coating or an imperfect finish, especially if the process isn’t carefully monitored. You might end up with a surface that’s rough, grainy, or too thick in some areas and too thin in others.
2. Reduced Quality of the Plating
As current increases, the grain structure of the metal being plated can become larger and rougher. This means that the plated layer might lose its smoothness and become more prone to defects. For example, you might notice pitting (small holes in the surface), blisters, or a shiny-but-soft surface. These defects result from the fact that high current can cause metal ions to be deposited unevenly, leading to poor adhesion and a rough finish.
Additionally, higher currents can promote the formation of hydrogen bubbles at the cathode (your substrate), which can be trapped within the plating layer. This leads to poor bonding and a reduction in the overall durability of the plated surface. So, while the plating may look good at first glance, it could fail over time due to these internal weaknesses.
3. Increased Risk of Heating
High current levels can generate excessive heat in both the electrolyte solution and the substrate. Electroplating baths are designed to operate within certain temperature ranges, and high current can push these temperatures above the optimal range. Excessive heat can cause several problems, such as:
- Thermal degradation of the electrolyte
- Expansion or warping of the substrate (especially in heat-sensitive materials)
- Disturbance of plating uniformity due to uneven cooling
This is especially problematic for more delicate substrates like plastic parts or thin metallic layers, which can warp or melt if exposed to excessive heat. If you’re working with a material that’s sensitive to heat, a high current could undo all of the careful work you’ve done in other aspects of the plating process.
4. Thicker Plating Layers
While high current leads to faster deposition, it can also cause thicker layers of plating. This can be beneficial in some situations where a thick, durable coating is required (e.g., in industrial or protective coatings). However, thick layers may suffer from:
- Cracking under stress
- Poor adhesion to the substrate, especially when plated too quickly
- Inconsistent thickness at different points on the substrate surface
If a thick coating is desired, it’s usually better to adjust the process to deposit the metal slowly but consistently, ensuring that each layer bonds well before the next one is added. Controlled current combined with other process parameters can provide thicker coatings without the problems associated with high current.
5. Side Effects Like Gas Formation
Excessive current can also lead to the production of gases, such as hydrogen gas, as a byproduct of the electroplating reaction. This gas formation typically occurs at the cathode, where the metal ions are reduced. The bubbles can adhere to the surface and cause issues with plating quality. They might:
- Disrupt the uniformity of the plating process
- Trap gas within the coating, weakening the adhesion
- Disturb the electrolyte’s composition, affecting the plating efficiency
While small amounts of gas are normal in the process, high currents tend to exacerbate this issue, and the resulting bubbles can lead to a less-than-ideal finish.
Is High Current Always Good for Electroplating? When Should You Avoid It?
As we’ve seen, high current can have both benefits and drawbacks in electroplating. But is it always a good idea to go for high current? In most cases, the answer is no. There are certain situations where high current should be avoided, or where a more controlled, lower current approach is better.
1. When Plating Delicate Materials
When plating delicate or heat-sensitive materials, such as plastics, thin films, or microelectronics, high current can be detrimental. The excessive heat generated by high current can cause the substrate to warp, crack, or even melt. Moreover, fast metal deposition may result in poor-quality finishes, with defects like pitting or gas bubbles ruining the smoothness and appearance of the layer. In these cases, lower current densities or pulse plating (a method that alternates between periods of high and low current) might be more suitable to achieve high-quality results.
2. Thin Coatings for Aesthetic Purposes
If your goal is to achieve a thin, fine finish—such as in the case of gold plating for jewelry or decorative items—high current isn’t your best friend. For thin coatings, a more controlled plating process with lower current is often preferred. This helps avoid the deposition of excess metal, ensuring the finish remains smooth and uniform.
3. When Consistency and Quality Matter Most
In applications where plating uniformity and quality are critical, such as in electronics or medical devices, high current can cause more harm than good. Here, the focus is often on precision, which requires precise control over the plating process. Higher currents can lead to uneven coating, especially on intricate or complex substrates. In these cases, it’s better to go with a controlled current for an even and consistent result.
At this point, we’ve explored how high current impacts electroplating, and whether it’s always the best option. But there are still more factors to consider, including when to use lower current, the ideal plating conditions, and how to achieve the best results in your electroplating process.
Factors to Consider When Deciding Whether High Current is Good for Electroplating
Now that we’ve discussed the benefits and potential pitfalls of using high current for electroplating, let’s dive deeper into the key factors you should consider when determining whether high current is the best option for your electroplating process. It’s not a one-size-fits-all scenario, and understanding the dynamics at play will help you make the right decision.
1. Type of Metal Being Plated
Different metals react differently to current during the electroplating process. Some metals require higher currents to achieve a smooth and durable finish, while others need a more gentle approach.
- Gold and Silver Plating: Both gold and silver electroplating generally require lower currents for fine, high-quality coatings. High current can cause grainy, rough deposits that are undesirable for aesthetic finishes.
- Nickel and Copper: These metals can tolerate slightly higher currents without compromising quality. Nickel plating, for example, can handle higher current densities if you want a thicker layer, but too much can lead to uneven coatings.
- Chrome and Zinc: Chrome plating often uses higher current densities for robust coatings, while zinc can sometimes require lower currents for finer finishes.
Each metal has its own optimal current density, which is a balance of current and time to achieve the desired plating thickness and finish. Researching specific plating guidelines for the metal you’re working with will help you identify the ideal current range.
2. Electrolyte Composition
The composition of the electrolyte—especially the concentration of metal salts—also affects how current will behave during electroplating. Some electrolytes can handle higher currents without deteriorating, while others might break down, leading to side reactions or defects in the plating process.
For example:
- Gold plating typically uses a cyanide-based electrolyte, which can tolerate higher currents but requires careful temperature and pH control.
- Nickel and copper plating often use acidic or acidic-neutral electrolytes, where high current can speed up the process but might also result in rougher surfaces if not controlled.
If the electrolyte is not suited to high currents, overloading the solution can lead to degradation, poor plating, and a shorter lifespan for your bath.
3. Plating Bath Temperature
The temperature of the electrolyte bath is closely tied to how well current can flow and how efficiently the electroplating process occurs. High current tends to generate heat, which could be both beneficial and detrimental depending on the circumstances.
- Higher temperatures (within the recommended range for your plating process) can accelerate the metal deposition rate, making high current a good option for faster plating.
- However, excessive heat caused by high current can cause thermal degradation of the electrolyte, leading to issues like uneven plating or poor adhesion.
To optimize current in relation to bath temperature, it’s important to have temperature control mechanisms in place, such as heating elements or cooling systems, to maintain the proper balance.
4. Substrate Material
The material being plated can also influence how well it interacts with high current. Some substrates, especially those made from heat-sensitive materials like plastics, might not withstand the high temperatures or rapid deposition speeds that come with high current.
Additionally, substrates with complex shapes or small features (like microelectronics) can be particularly sensitive to current density. High current in these cases might result in poor uniformity or excessive buildup in certain areas of the substrate.
For example, while flat metallic substrates may handle high current without much issue, intricate parts or delicate shapes may require lower current and more precise control to avoid uneven plating or damage.
5. Equipment and Power Supply Capabilities
Not all electroplating equipment is designed to handle high currents. When deciding whether to use a higher current, it’s essential to ensure that your equipment, especially the power supply or rectifier, can deliver the necessary current and manage the heat generated.
For industrial-scale electroplating, rectifiers are used to convert alternating current (AC) into direct current (DC), which is necessary for electroplating. These rectifiers often come with adjustable current settings to fine-tune the process. However, not all equipment can handle higher currents for extended periods. Overloading your system could cause equipment failure or result in inefficient plating.
What is the Ideal Current for Different Electroplating Processes?
Now that we understand the factors to consider, let’s go over the ideal current ranges for specific electroplating processes. These ranges depend on the type of metal, the desired outcome, and the plating bath conditions. Understanding the proper current for each process will help you avoid issues like uneven deposits or poor adhesion.
1. Gold Electroplating
- Ideal Current Density: Typically between 0.1 – 1 A/dm² (amperes per square decimeter)
- Why it matters: Gold plating is often used for aesthetic or electronic applications, requiring a smooth, fine finish. Higher currents can result in rougher deposits, which aren’t suitable for high-quality gold finishes.
- Tip: Use low current for thin, decorative layers and high pulse plating for more uniform and smoother finishes.
2. Nickel Electroplating
- Ideal Current Density: 2 – 4 A/dm² for a standard nickel finish, up to 6 A/dm² for heavy plating
- Why it matters: Nickel is often used for protective coatings, like corrosion resistance. Higher currents are more suitable when a thicker and more durable layer is needed, but be cautious of too much current, which can lead to rougher finishes.
- Tip: For a smooth, fine finish, stay closer to the lower end of the current range.
3. Silver Electroplating
- Ideal Current Density: 0.5 – 2 A/dm²
- Why it matters: Silver electroplating requires a smooth, shiny finish. Excessive current can result in grainy deposits that detract from its high-luster look.
- Tip: For fine silver finishes, use lower current and adjust the electrolyte conditions for optimal results.
4. Copper Electroplating
- Ideal Current Density: 1 – 3 A/dm²
- Why it matters: Copper plating is often used for thick coatings and electrical conductivity, where high current can help speed up the process. However, the key is maintaining a balanced current to prevent issues like uneven deposition.
- Tip: For uniform coatings, it’s important to control both current and bath agitation to ensure even coverage.
5. Chrome Electroplating
- Ideal Current Density: 2 – 6 A/dm² depending on the thickness and type of chrome plating (hard chrome vs decorative chrome)
- Why it matters: Chrome plating can benefit from higher current for a hard, durable finish, especially in industrial applications. However, decorative chrome should be plated with a more controlled current to avoid defects.
- Tip: Use a moderate current for decorative finishes and higher current for heavy-duty industrial chrome plating.
How to Control and Optimize Current for Electroplating?
Now that you know the ideal current for different processes, the next step is optimizing and controlling the current to ensure the best electroplating results. Here are some key tips and techniques to help you achieve consistent, high-quality plating:
1. Use Pulse Plating
Pulse plating is a technique that alternates between high and low currents. This helps reduce the negative effects of high current (like rough deposits and hydrogen gas production), while still achieving fast deposition rates. It allows you to control the plating process more precisely and is particularly useful for metals that tend to produce poor results under continuous high current.
2. Monitor Current and Voltage Regularly
Using a rectifier with adjustable settings lets you monitor the current density throughout the electroplating process. Keep an eye on both voltage and current to ensure they stay within the optimal ranges for your specific application. Regular monitoring will help you identify potential issues early on and make adjustments as needed.
3. Adjust for Bath Temperature
Make sure the temperature of your plating bath is within the recommended range for your specific metal. If you’re using high current, you may need to adjust the temperature to compensate for the additional heat generated by the current. Too much heat can lead to poor adhesion and inconsistent plating.
4. Experiment and Test
Every electroplating job is different, so experimentation is often necessary. Start with the recommended current range for your metal and adjust based on your observations. Testing different current densities on small samples can help you find the sweet spot for your process, ensuring that the final result meets your expectations.
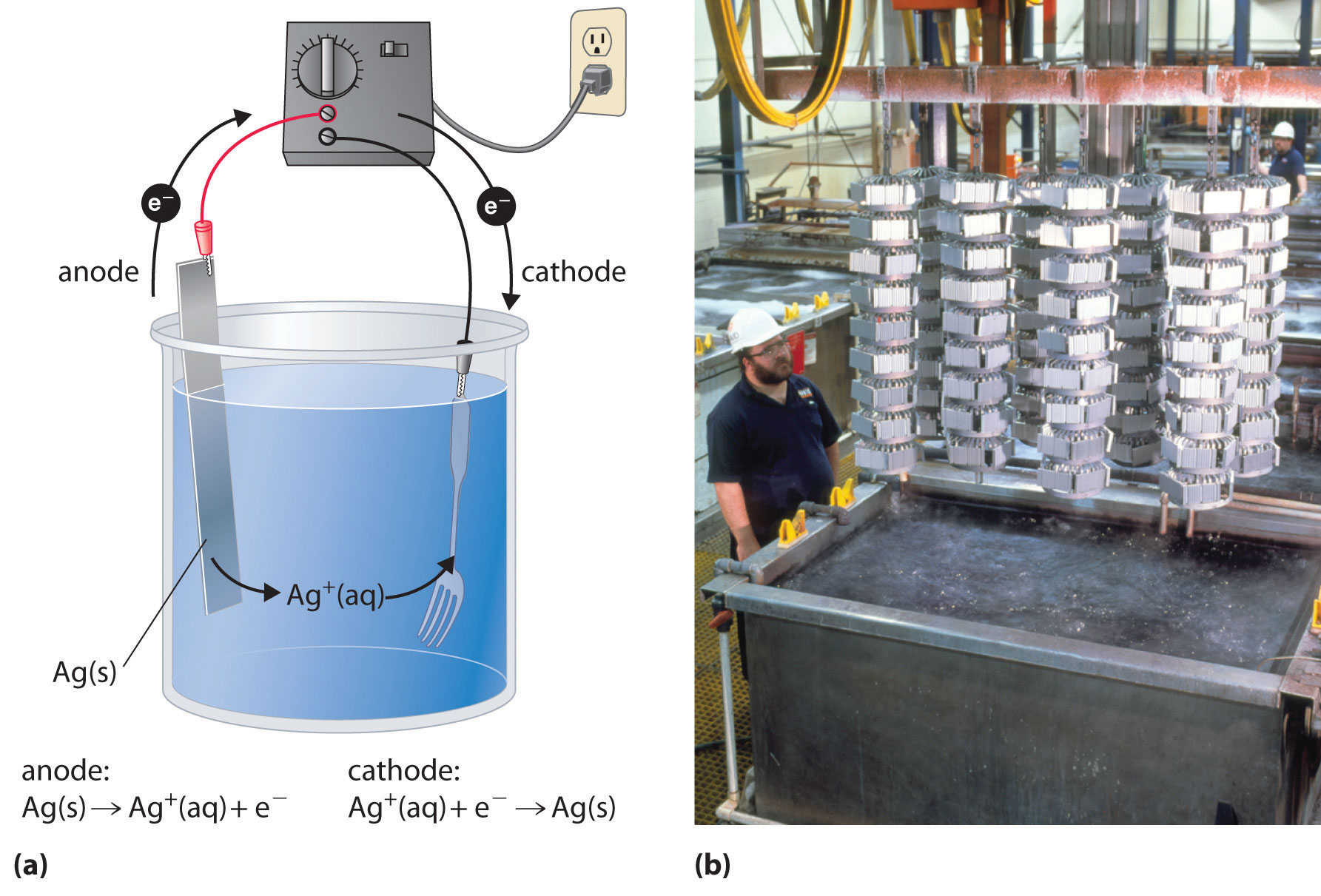
Common Mistakes When Using High Current in Electroplating
Electroplating, like any precise manufacturing process, requires attention to detail and careful monitoring. Using high current in electroplating can sometimes lead to mistakes that negatively impact the final result. Here are some common mistakes to watch out for, and how to avoid them:
1. Overloading the Electroplating Bath
One of the most common mistakes when using high current is overloading the electroplating bath, meaning applying more current than the electrolyte can handle. This can lead to several problems:
- Degradation of the electrolyte: High current can break down the electrolyte solution more quickly, reducing its effectiveness. Over time, this will affect the plating quality, causing uneven or poor deposition.
- Increased side reactions: Excessive current can cause unwanted side reactions, such as hydrogen gas production, which can result in bubbles on the plated surface. This disrupts the uniformity of the plating and leads to defects like pits and cracks.
Tip: Always follow the recommended current density for the specific electrolyte you’re using and avoid pushing it beyond the manufacturer’s guidelines.
2. Plating Too Quickly
Plating too quickly might seem like a good idea, especially if you’re in a rush to get the job done. But high current speeds up the deposition process, and while this may produce thicker coatings, it often results in poor-quality finishes.
- Uneven deposition: High current can lead to uneven plating, particularly in intricate designs or parts with sharp edges, where the current is concentrated. The metal might accumulate more in certain areas and less in others.
- Rough surface texture: A rapid deposition can cause the metal to form larger crystals, leading to a rough and uneven surface finish. This is especially problematic when you’re aiming for a smooth, shiny surface.
Tip: Instead of rushing, take the time to apply a moderate current and allow the process to unfold gradually. If speed is important, pulse plating can be an effective technique.
3. Inconsistent Current Control
Another common mistake is poor control over the current. This often happens when there is a lack of monitoring tools or failure to adjust the settings during the plating process.
- Current surges: Sudden surges in current can cause instant plating defects, such as blisters, cracks, or pitting. These defects typically arise from insufficient regulation of the power supply.
- Overheating: Without proper monitoring, the current can generate excessive heat, affecting both the plating bath and the substrate. This can cause thermal degradation, as we mentioned earlier, leading to warping or poor adhesion.
Tip: Invest in reliable rectifiers with adjustable settings and monitor current and voltage regularly to avoid fluctuations and surges.
4. Not Accounting for Substrate Material
Different materials react differently to electroplating, and high current is not always suitable for all types of substrates. For example, plating onto a plastic substrate requires careful control of both current and temperature to prevent warping or cracking. Using high current on materials that are sensitive to heat can result in disastrous effects.
Tip: Always tailor the current to the material being plated, and consult plating guidelines specific to the substrate you’re working with. If plating delicate or heat-sensitive materials, consider using lower current and ensuring proper cooling.
5. Ignoring Bath Agitation
Agitation is an often-overlooked factor in electroplating. Without proper bath agitation, the electrolyte might not be evenly distributed across the substrate, leading to an uneven deposition rate, especially with high current.
- Uneven coating: High current can concentrate in certain areas of the surface, but if the electrolyte isn’t circulating properly, some parts of the substrate may receive too little metal, while others get too much.
- Gas accumulation: Inconsistent agitation can also allow hydrogen gas to accumulate in certain areas, affecting the uniformity of the coating.
Tip: Ensure that the bath is agitated properly, either manually or using mechanical or air agitation systems. This helps in achieving a uniform distribution of current and metal ions.
Alternative Methods to Improve Electroplating Without Increasing Current
If high current is not the best solution for your electroplating needs, there are alternative methods you can use to improve plating quality and efficiency without relying on excessive current. Here are some strategies:
1. Pulse Plating
Pulse plating is a technique that alternates between high and low current, creating pulses of energy rather than a continuous flow. This helps overcome many of the drawbacks of high current:
- Improved surface quality: Pulse plating reduces the occurrence of rough, grainy deposits and improves adhesion by allowing the plating to occur more smoothly.
- Reduced heat buildup: Because the current is not continuous, the heat generated is less intense, minimizing the risk of overheating the electrolyte or substrate.
- Control over deposition rate: The periodic breaks between pulses allow for more precise control over the plating process, helping you achieve uniform coating thickness and finer finishes.
Pulse plating is particularly useful for applications that require high-quality finishes, such as electronics and jewelry, where the appearance and durability of the plating are paramount.
2. Adjusting Electrolyte Composition
The composition of your electrolyte solution plays a significant role in the quality of the electroplating process. By optimizing the electrolyte composition, you can achieve smoother, more uniform deposits, even at lower current densities.
- Additives and brighteners: Adding specific chemicals (like brighteners, levelers, or complexing agents) to the electrolyte can improve the quality of the plating without increasing the current. These additives can help smooth out the plating surface, prevent defects, and enhance the overall appearance of the finish.
- Bath pH and temperature: Maintaining the right pH and temperature can improve the efficiency of the electroplating process, helping to produce higher-quality coatings without the need for high current.
3. Optimizing Bath Temperature
Bath temperature can have a significant effect on the electroplating process. For example, increasing the temperature can speed up the deposition rate without increasing the current. However, you need to strike the right balance, as too high a temperature can cause problems like gas formation or electrolyte degradation.
- Cooler baths can help slow the process and allow for a more controlled deposition, while warmer baths can help the metal ions to more easily reach the substrate.
4. Increasing Time Rather Than Current
Sometimes, the best way to achieve a thicker coating or higher-quality finish is by increasing the plating time rather than the current. By plating at a lower current density over a longer period, you can build up the coating gradually without introducing defects. This approach is particularly useful when plating metals like gold or silver, where a smooth, fine finish is crucial.
Is High Current Good for Electroplating?
So, is high current good for electroplating? It depends. While high current can speed up the plating process and help achieve thicker coatings, it’s not always the best approach. High current can lead to rough finishes, uneven deposition, gas formation, and excessive heat, all of which can harm the final product.
The key takeaway here is that balance is essential. High current is suitable for some applications, like when plating durable layers of nickel or chrome, but for others, especially when precision and finish quality are paramount, lower currents or alternative techniques like pulse plating are preferable. Always consider factors like the type of metal, the substrate material, the electrolyte composition, and the desired finish when deciding whether to increase current.
By understanding the role of current and experimenting with different parameters, you can optimize your electroplating process for better results, improved quality, and greater efficiency.