Electroplating is one of those processes that we come across every day without thinking much about it. Whether it’s the shiny chrome on your car’s bumper or the gold-plated jewelry you wear, electroplating plays a huge role in giving materials that glossy, durable finish. But if you’re here, you probably have one big question on your mind: Is electroplating physics or chemistry? The truth is, it’s both—and understanding why involves diving into some fundamental principles of both disciplines.![]()
Electroplating refers to the process of depositing a thin layer of metal onto the surface of an object through the use of electricity. This method is widely used in various industries to enhance the properties of the base material, whether it’s for aesthetic reasons (think shiny, gold-plated jewelry) or functional reasons (such as improving durability, corrosion resistance, or electrical conductivity).
The electroplating process involves an electrical current that causes metal ions in a solution to be reduced and deposit onto a substrate material (often called the “cathode”). The metal used in the plating process can vary, but common examples include gold, silver, copper, nickel, and chrome. Electroplating allows manufacturers to coat a wide variety of items, ranging from household items and industrial components to cutting-edge electronics and automotive parts.
Why is Electroplating Important?
Now, you might be wondering, “Why go through all the trouble of electroplating in the first place?” Here are a few reasons why electroplating is so widely used:
- Improved Durability: By adding a layer of metal to a surface, electroplating can significantly enhance its resistance to wear and tear, corrosion, and tarnish.
- Aesthetic Appeal: Electroplated items often look more polished and shiny, which is why it’s commonly used in jewelry and decorative items.
- Enhanced Conductivity: In electronics, electroplating can provide a layer of metal that improves the flow of electricity between parts.
- Cost Efficiency: By electroplating, manufacturers can coat inexpensive materials (like plastic or copper) with expensive metals (like gold or silver), reducing overall material costs while maintaining quality and appearance.
How Does Electroplating Work?
A Simple Explanation of the Process
At its core, electroplating is an electrochemical process. It uses an electrical current to drive a chemical reaction that deposits metal ions onto the surface of a substrate. Here’s a step-by-step breakdown:
- Preparation: The object to be electroplated (called the cathode) is cleaned and prepared. This step is crucial because any dirt, oil, or oxidation on the surface can prevent the metal from adhering properly.
- Electrolyte Solution: The object is then submerged in an electrolyte solution, which contains metal salts of the metal you want to plate onto the surface. For example, if you’re plating with gold, the solution might contain gold chloride or gold cyanide.
- Applying the Current: A direct current (DC) is passed through the electrolyte. The metal in the electrolyte is in an ionic form, and the electrical current causes these ions to move toward the cathode (the object being plated).
- Deposition of Metal: When the metal ions reach the cathode, they gain electrons and are reduced into solid metal, which then adheres to the surface of the object.
- Completion: Over time, the metal layer builds up, creating the final electroplated coating.
The result? A smooth, shiny, and durable surface, depending on the metal used and the quality of the electroplating process.
Is Electroplating a Chemical or Physical Process?
Addressing the Core Question: Is Electroplating Physics or Chemistry?
This is where things get interesting. To answer the question, “Is electroplating physics or chemistry?”, we have to break it down:
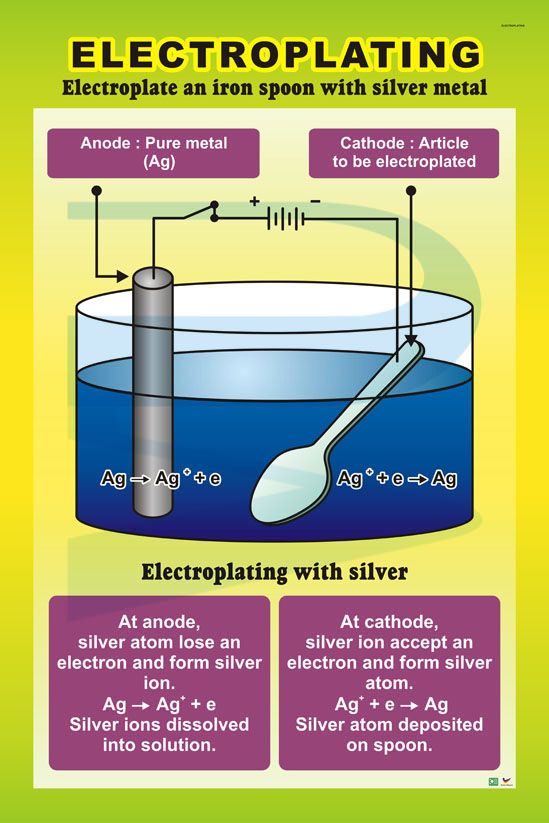
- Chemistry: Electroplating is definitely rooted in chemistry. The whole process hinges on chemical reactions. Specifically, the oxidation of the metal at the anode (positive electrode) and the reduction of metal ions at the cathode (negative electrode) are purely chemical processes. These redox reactions are what drive the deposition of metal onto the object you want to plate.
- Physics: But don’t be fooled into thinking that chemistry is all there is to it. The process wouldn’t happen without the physical principles of electricity and magnetism. When the electrical current is passed through the electrolyte, it’s the physics of electric fields and current flow that govern how the metal ions move and adhere to the surface. The voltage, current, and the distance between the electrodes all play a physical role in determining how well the plating adheres and how thick or smooth the coating will be.
So, to put it simply: electroplating is both chemistry and physics in action. You can’t have one without the other—both fields come together to make the process work smoothly and effectively.
Electroplating is a Beautiful Mix of Chemistry and Physics
We’ve learned that electroplating involves both chemical reactions and physical principles. The chemistry controls the reactions that reduce metal ions onto the object’s surface, while the physics governs the movement of those ions and ensures they’re deposited evenly. If you want to fully understand electroplating, you can’t ignore either side. It’s a perfect example of how both science fields work hand in hand to create something amazing.

The Role of Chemistry in Electroplating
The Chemical Reaction Behind Electroplating
When we talk about electroplating being chemistry in action, the core of it lies in the electrochemical reactions that occur during the process. These reactions are what allow metal ions to be deposited onto the surface of the object being plated. Here’s a more detailed look at the chemical processes involved:
- Oxidation at the Anode (Positive Electrode):
- The anode (the electrode connected to the positive side of the power supply) is made from the metal that you want to plate onto the object. As the electric current passes through the electrolyte, the metal at the anode undergoes oxidation (losing electrons) and dissolves into the solution as positive metal ions.
- For example, if you are electroplating with gold, the gold anode will dissolve into the electrolyte as gold ions (Au³⁺).
- Reduction at the Cathode (Negative Electrode):
- At the cathode, which is the object you want to plate, reduction occurs. This is where the metal ions from the electrolyte gain electrons and are converted into solid metal, which then adheres to the surface of the object.
- So, in the case of gold electroplating, the gold ions in the solution will be reduced (gain electrons) and become gold atoms, which then deposit onto the surface of the object.
Choosing the Right Electrolyte Solution
The electrolyte solution is crucial in electroplating because it contains the metal ions needed to create the plating. The composition of the solution affects the quality of the electroplated layer, including its texture, thickness, and adhesion.
- Metal Salt: The electrolyte typically contains a salt of the metal you’re plating. For example, for gold electroplating, gold chloride (AuCl₃) or gold cyanide (Au(CN)₂⁻) are commonly used as the electrolyte.
- Additives: Various chemicals are added to improve the plating quality. These can include brighteners to create a smooth, shiny surface, or inhibitors to prevent unwanted reactions.
- pH Control: The pH level of the solution plays a significant role in how well the metal deposits onto the object. Too high or too low a pH can lead to poor plating results. For example, gold electroplating typically occurs at a slightly acidic pH.
The Impact of pH, Temperature, and Concentration on Electroplating
- pH: The pH level of the electrolyte affects the nature of the plating. A slightly acidic solution can help in the smooth deposition of metals, while highly acidic or alkaline solutions can cause rough or uneven plating.
- Temperature: The temperature of the electrolyte also plays a significant role in the speed and quality of the plating. Higher temperatures generally increase the reaction rate, but too high a temperature can lead to unwanted side reactions.
- Concentration: The concentration of metal ions in the solution will influence how thick or smooth the plated layer becomes. A low concentration may lead to slower deposition, while a high concentration can lead to faster plating, but with potential for uneven coating.
These chemical variables need to be carefully controlled to ensure high-quality electroplating. A slight imbalance in one of these factors can result in poor adhesion, uneven surfaces, or other issues in the final product.
The Role of Physics in Electroplating
The Physics of Electric Current in Electroplating
As we established, chemistry is essential in driving the reactions that allow metal to coat the object, but physics governs how those reactions happen. When we talk about the physics involved, we’re referring to the way electric current and voltage influence the electroplating process.
How Does Electric Current Affect Electroplating?
- Electricity and Ion Movement:
- The primary role of electricity in electroplating is to create an electric field within the electrolyte solution. This field is what causes the positively charged metal ions to move toward the cathode (the object being plated).
- Without electricity, the metal ions wouldn’t have the energy to move and get reduced onto the object’s surface.
- Current Density:
- Current density refers to the amount of current flowing per unit area of the cathode. The amount of current you apply will directly affect how thick and smooth the electroplated layer becomes. Too much current can cause the metal to deposit too quickly, creating rough, uneven layers.
- On the other hand, too little current can cause the plating process to be too slow, resulting in weak, inconsistent plating. Achieving the right current density is key to getting a high-quality finish.
- Voltage:
- Voltage (the electric potential difference) also plays a vital role in electroplating. A higher voltage will increase the rate of the electroplating process, but if the voltage is too high, it can lead to excessive heat, poor adhesion, or unwanted side reactions.
- Adjusting the voltage allows the technician to control how fast the metal is deposited and how smooth the surface becomes.
The Influence of Electric Fields and Magnetic Fields
- Electric Fields: In electroplating, the electric field created by the applied voltage helps direct the flow of metal ions in the electrolyte solution. The ions are attracted to the cathode due to this field, but how they move depends on the strength of the field and the distance between the electrodes.
- Magnetic Fields: In some cases, magnetic fields are used to improve the plating process. This is known as magnetic-assisted electroplating, and it can help achieve better uniformity and even plating over complex or intricate surfaces. By using magnetic fields, manufacturers can ensure that the metal ions reach every part of the object, regardless of shape.
How the Physical Properties of the Substrate Affect Electroplating
The physical characteristics of the object you’re plating also play a significant role in the electroplating process. These factors include:
- Conductivity: For electroplating to work, the surface must be conductive enough to carry the electric current. Metals like copper and silver are excellent conductors, which is why they are often used as substrates for electroplating. Non-conductive materials, like plastics, can be electroplated using a special process to make them conductive first.
- Surface Texture: The texture of the substrate surface affects how well the metal will adhere. A rough surface may cause the plating to form unevenly, while a smooth, clean surface allows for more uniform deposition of metal.
- Temperature: The temperature of both the substrate and the electrolyte solution will impact how well the electroplated metal adheres. If either is too hot or too cold, the plating may be weak or poorly adhered.
Electroplating is Both Chemistry and Physics
As we’ve explored, electroplating involves both chemical and physical processes working together. The chemical side governs the redox reactions that make the plating possible, while the physical side deals with electricity, current, voltage, and the physical properties of the materials. Both disciplines are essential to ensure a smooth, durable, and high-quality electroplated finish.
Now that we’ve explored the science behind electroplating, you can appreciate the complexity and beauty of the process, as well as how chemistry and physics combine to produce these stunning results. Whether you’re a professional in the field or someone who simply appreciates the shiny, durable objects electroplating produces, understanding both sides of the process is key to truly grasping how it all works.
/battery-connected-to-a-copper-pipe-and-a-key-in-copper-sulphate-solution-copper-plating-dor20023034-57a48f613df78cf459fe179c.jpg)
Why Electroplating is a Combination of Both Physics and Chemistry
The Integration of Chemical and Physical Principles
As we’ve seen, electroplating is a process that blends both chemistry and physics. It’s not just about the metal ions floating around in a solution, nor is it just about electric currents passing through the electrolyte. To achieve optimal electroplating results, you need to combine the understanding of chemical reactions and the principles of physics.
Why is this integration so important?
- Chemical Reactions: The heart of the electroplating process lies in the chemical reaction between metal ions and the electrons provided by the electrical current. It’s these oxidation and reduction reactions that facilitate the plating. Without a solid understanding of how these reactions work, it would be impossible to control the metal deposition effectively.
- Physics of Electricity: The role of electricity is just as crucial. The electric current ensures the metal ions are pulled to the cathode and deposited uniformly on the object. Factors like voltage, current density, and electric fields all impact the physical aspects of electroplating. A proper understanding of electricity and magnetism allows for better control of the plating process.
For example, let’s take current density—it’s a physics-based factor, but it also influences the chemical reaction. A higher current density might cause more rapid deposition, but it could result in poor adhesion or uneven surfaces. Understanding the relationship between chemical reactions and physical forces is essential for a successful plating process.
Electroplating and Its Dependence on Both Fields
The importance of both physics and chemistry is even more apparent when you look at the way fine-tuning the process can drastically improve results. Adjusting voltage (a physical factor) can lead to smoother plating, while altering the electrolyte composition (a chemical factor) can influence the rate of deposition and overall quality.
To make it simple, think of electroplating as a two-part recipe: chemistry provides the ingredients (metal ions and solutions), and physics controls the cooking time and temperature (electric current and voltage). Without either one, the process wouldn’t work as efficiently or effectively.
Key Factors That Influence the Electroplating Process
How Voltage and Current Affect the Plating Outcome
One of the most significant factors in the electroplating process is voltage and current. These factors control the rate at which metal is deposited and can even influence the quality of the finished product.
- Voltage: Higher voltage typically speeds up the electroplating process, which can sometimes lead to thicker coatings. However, if the voltage is too high, it may cause excessive heat or irregular plating. The trick is finding that sweet spot where voltage is just high enough to ensure smooth plating but not so high that you run into issues with quality.
- Current Density: Current density is a direct result of the voltage applied, and it’s one of the most important factors in determining the thickness and uniformity of the metal layer. Higher current density can result in faster plating but may cause rougher surfaces. On the other hand, a lower current density produces smoother finishes but can take longer.
A balance of both voltage and current ensures that the electroplating process happens efficiently, with the right amount of metal deposition and the best surface quality.
The Importance of Time in Electroplating
Time is also a crucial factor in electroplating, as it influences the thickness of the metal layer. A short plating time might result in a thin, fragile layer, while longer plating time can result in a thicker, more durable coating.
- Short Plating Time: When plating is completed too quickly, it may result in a less durable layer that could easily peel or corrode over time.
- Longer Plating Time: On the other hand, longer plating times can lead to thicker layers, but this could sometimes result in unwanted surface imperfections, such as bubbles or streaks.
Controlling the time in the electroplating process ensures that the layer is of the desired thickness, providing the perfect balance between strength and aesthetic appeal.
Temperature and its Effects on the Plating Process
Temperature is another factor that has a direct impact on electroplating. If the electrolyte solution gets too hot, it can increase the rate of deposition, but too much heat could cause uneven plating and even damage the material you are plating. On the other hand, if the solution is too cold, the process can slow down and result in poor adhesion.
- Optimal Temperature Range: Electroplating is typically carried out at moderate temperatures (around 40–60°C), but the ideal temperature varies depending on the type of metal being plated.
By carefully controlling the temperature, manufacturers can ensure a uniform, high-quality electroplated layer with better adhesion.
Common Electroplating Techniques
Different Electroplating Methods
While the basic electroplating process is quite similar across different metals, there are several techniques that are used for specific needs:
- Galvanization: This is a common method used to plate steel or iron with a layer of zinc. The primary purpose of galvanization is to protect the metal from corrosion.
- Electroless Plating: Unlike traditional electroplating, which requires electricity, electroless plating uses a chemical solution to deposit metal onto an object. This method is often used for materials that cannot conduct electricity, such as plastics.
- Hard Chrome Plating: This process uses chromium to create a hard, durable coating on metal surfaces, often used in tools and machinery to increase wear resistance.
Each of these techniques has its own set of rules and requirements, but all of them rely on both chemistry and physics to ensure a high-quality finish.
What Materials are Commonly Electroplated?
Electroplating can be used to plate a wide variety of metals. The most commonly electroplated metals include:
- Gold: Used in jewelry, electronics (like connectors and circuit boards), and high-end consumer products. Gold plating enhances both the appearance and durability of objects.
- Silver: Known for its shiny appearance, silver is often used in jewelry, tableware, and electronics.
- Nickel: Used for its corrosion resistance and hard surface properties. Nickel plating is common in automotive parts and machinery.
- Copper: Copper electroplating is widely used for electrical applications like wires and printed circuit boards.
- Chrome: Often used in automotive parts, chrome plating provides a glossy, shiny finish and excellent corrosion resistance.
Applications of Electroplating in Different Industries
Electroplating in the Automotive Industry
In the automotive industry, electroplating plays a key role in enhancing both the aesthetic appeal and durability of car parts. Chrome plating is especially popular in creating sleek, shiny finishes for bumpers, wheels, and trims. Electroplated coatings also provide resistance to corrosion, keeping car parts looking new longer.
Electroplating in Electronics
Electroplating is also widely used in the electronics industry. Gold and silver are often plated onto connectors, circuit boards, and other components to improve electrical conductivity and prevent corrosion. Gold-plated connectors are particularly important for high-end electronics like smartphones, where reliable connections are a must.
Electroplating in Jewelry Making
Perhaps one of the most well-known uses of electroplating is in the jewelry industry. Gold, silver, and rhodium are commonly used to plate jewelry, giving it a shiny finish and improving its appearance. The process allows jewelers to use less of the precious metals while still achieving a high-quality look.
Electroplating in Medical Devices
Medical devices, such as surgical instruments or implants, often undergo electroplating to improve their corrosion resistance and wear resistance. This ensures that the devices maintain their integrity over time and provide the necessary durability for medical use.
Is Electroplating More Chemistry or Physics?
Why Both Chemistry and Physics Matter in Electroplating
We’ve covered a lot of ground in exploring the question, “Is electroplating physics or chemistry?” The final answer is clear: it’s both. While the process is driven by chemical reactions that enable metal deposition, the physical principles of electricity and current flow are just as crucial in determining the quality and consistency of the plating.
Electroplating truly exemplifies how chemistry and physics can come together to create something remarkable. Understanding the interplay between both fields is essential for anyone working with or studying electroplating processes, as it allows for better control, improved outcomes, and a deeper appreciation for this fascinating technology.
FAQs About Electroplating
What is the Difference Between Electroplating and Galvanization?
Although electroplating and galvanization might seem similar at first, they serve slightly different purposes and involve different metals:
- Electroplating: This is a broader term that can refer to plating any metal onto another, and the process is primarily used to improve the appearance, durability, or functionality of a wide variety of objects. Common metals used in electroplating include gold, silver, nickel, chrome, and copper.
- Galvanization: This specific type of electroplating involves coating iron or steel with a layer of zinc to protect it from rust and corrosion. The main purpose of galvanization is to protect the metal from the elements, making it ideal for outdoor equipment, construction materials, and car parts exposed to moisture.
Galvanization is a form of electroplating but is specifically used for corrosion protection with zinc as the metal used for plating.
Can Electroplating Be Done Without Electricity?
Yes, electroplating can be done without electricity, but the process is known as electroless plating. Unlike traditional electroplating, which uses an electric current to deposit metal ions onto an object, electroless plating relies on chemical reactions to deposit the metal.
- In electroless plating, a chemical bath is used to reduce metal ions in the solution and deposit them onto the substrate. The absence of electricity in this process makes it ideal for plating non-metallic materials like plastic. It’s commonly used for applications like nickel plating and gold plating on non-metallic substrates.
Electroless plating offers advantages such as more uniform plating on complex shapes, and it can be applied to materials that cannot conduct electricity (such as plastics).
What Are Some Common Problems in Electroplating?
While electroplating is a highly efficient process, it’s not without its potential challenges. Some of the most common issues in electroplating include:
- Poor Adhesion: If the metal coating doesn’t stick properly to the substrate, it may peel or flake off over time. This could happen if the surface wasn’t properly cleaned, or if the plating conditions (voltage, current, pH) weren’t optimal.
- Uneven Plating: Uneven plating can result from issues like improper current distribution, poor electrolyte agitation, or incorrect distance between the electrodes. This can cause areas to receive more metal than others, resulting in rough or patchy plating.
- Bubbles and Defects: Bubbles, pinholes, or other defects in the plated layer can occur if the plating bath is contaminated, the temperature is too high, or the current is too strong. These defects can affect both the appearance and functionality of the plated item.
- Excessive Thickness: While a thicker plating might seem like a good thing, it can sometimes lead to poor adhesion or cracking if the coating is too thick. Finding the right balance in plating thickness is crucial.
The key to avoiding these problems is careful monitoring of plating conditions, including current density, voltage, temperature, and electrolyte composition.
What Is the Future of Electroplating Technology?
The future of electroplating is promising, with advancements in both technology and materials. Here are a few trends that could shape the future of electroplating:
- Green Electroplating: The traditional electroplating process often involves toxic chemicals like cyanide and chromium, which can pose environmental risks. However, green electroplating technologies are emerging that use more environmentally friendly chemicals, which are safer for both workers and the environment. Non-toxic plating solutions and waste minimization techniques are key areas of innovation.
- Nanotechnology: The use of nanomaterials in electroplating could enable the creation of coatings with new properties—such as improved strength, conductivity, or resistance to wear. Nanocoatings could be used in everything from electronics to medical devices, offering new opportunities for precision and durability.
- Automation and AI in Electroplating: The application of automation and artificial intelligence (AI) in electroplating could lead to more precise control of the plating process. AI systems could monitor variables like current density, voltage, and temperature in real-time to optimize plating conditions, improving efficiency and consistency.
- Electroplating for Advanced Manufacturing: As industries like aerospace and electronics push the boundaries of performance, electroplating techniques will evolve to meet these new challenges. Electroplating will likely play a role in creating lightweight, high-performance coatings for parts used in extreme environments.
With these innovations, electroplating will continue to be a critical part of manufacturing, helping industries create products that are more durable, functional, and environmentally friendly.
Is Electroplating More Chemistry or Physics?
We’ve now come full circle. The short answer to the question, “Is electroplating physics or chemistry?” is that it’s both. The science behind electroplating is a perfect blend of chemistry and physics. While the chemical reactions are what make the process possible, the physical principles of electricity and current flow are what shape how the plating is applied.
By mastering both the chemistry of the electrolyte solution and the physics of the electric current, we can ensure high-quality electroplating that results in durable, functional, and aesthetically pleasing finishes. Whether you’re in the automotive, electronics, jewelry, or medical industries, electroplating is a critical process that combines scientific disciplines to create products that enhance our daily lives.
As technology advances, electroplating will only continue to evolve, embracing greener technologies, smarter systems, and more advanced materials to meet the demands of the future.