Electroplating might sound like something straight out of a sci-fi movie, but it’s actually a well-established process with practical applications everywhere—from the jewelry you wear to the chrome finishes on your car. So, what is it, really?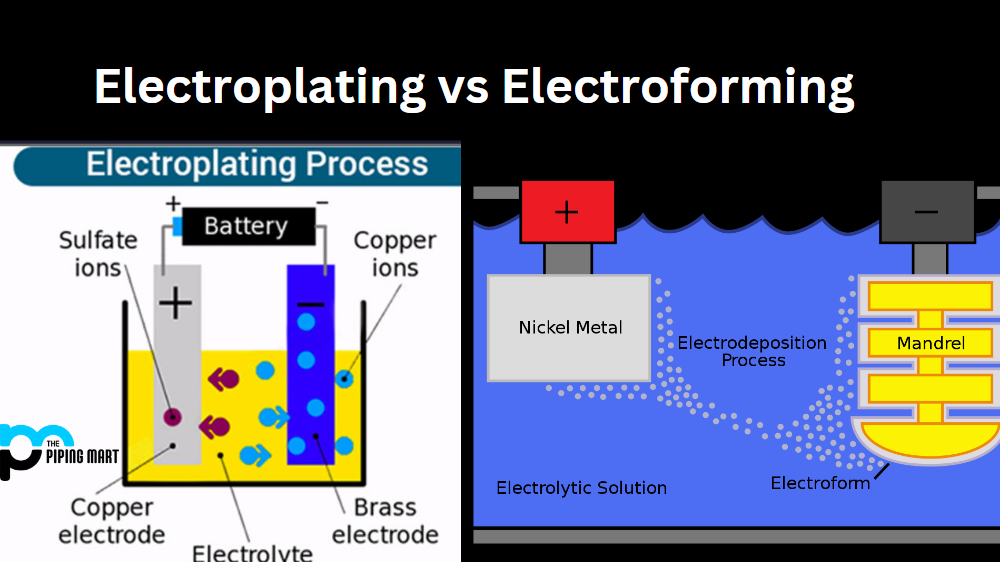
Electroplating is a process where a thin layer of metal is deposited onto the surface of another material using an electric current. Essentially, it’s a way to coat objects with metals like gold, silver, copper, nickel, or chrome, giving them a shiny finish, better durability, or even resistance to rust. It’s like giving an object a metallic “makeover,” except this makeover isn’t just skin-deep (well, maybe it’s layer-deep).
The materials most commonly electroplated are metals, but other surfaces like plastics or ceramics can be plated too—though the process is a little trickier. You might encounter electroplated items every day without even realizing it, from gold-plated rings to nickel-plated kitchen faucets.
How Electroplating Works
Imagine this: you’ve got a fancy metal object (or, more likely, a not-so-fancy object you want to make fancier), and you want it coated with a shiny new layer of metal. Electroplating is the magic that makes it happen, and here’s how it works:
- Preparation: The surface to be plated is thoroughly cleaned. Any dirt, grease, or oxidation has to go—metal needs a “clean slate” for plating to stick properly.
- The Setup: The object to be plated (the cathode) is submerged in a solution containing dissolved metal salts (the electrolyte). The metal you’re coating with acts as the anode.
- The Electric Current: When an electric current is passed through the solution, metal ions from the anode move through the electrolyte and deposit onto the cathode. Voilà! A thin, shiny layer begins to form.
- Final Touches: Once the desired thickness is achieved, the object is removed, rinsed, and dried. Sometimes, additional polishing or protective coatings are applied to enhance durability.
This process isn’t just for aesthetics, either. Electroplating is often used to improve corrosion resistance, conductivity, or even to repair worn-out parts by adding material back.
Common Applications of Electroplating
Electroplating might be fancy, but it’s far from rare. In fact, you’ve likely encountered its handiwork multiple times today. Here are some common areas where electroplating shines (sometimes literally):
- Jewelry: That affordable gold-plated necklace? Yep, it’s electroplated. Jewelry makers use this technique to create luxury looks without the hefty price tag.
- Automotive Industry: Those shiny chrome trims and bumpers on classic cars? Electroplated. This helps enhance aesthetics and protect surfaces from corrosion.
- Electronics: Ever wondered why connectors, circuit boards, or certain electronic components seem so shiny? Electroplating with gold or silver improves conductivity and prevents oxidation.
- Household Items: Think bathroom fixtures, kitchen faucets, or even decorative handles. Electroplating helps these items resist rust while maintaining their luster.
- Industrial Parts: In heavy industries, electroplating is used to extend the lifespan of machine components by making them harder and more resistant to wear.
Fun Fact:
The first documented use of electroplating dates back to the 19th century, when scientists discovered the process could make cheap materials look like precious metals. Imagine their excitement—instant alchemy!

Is Electroplating Permanent?
This is the million-dollar question—is electroplating permanent? The short answer is: not exactly, but it’s not as fleeting as you might think either. While electroplating is durable and long-lasting under the right conditions, it’s not truly “forever.” Let’s dive deeper into what permanence means in the world of electroplating.
What Does “Permanent” Mean in Electroplating?
When we talk about permanence, we’re usually asking, “Will this last forever without wearing off?” Unfortunately, electroplating doesn’t fit that description. Over time, factors like wear and tear, exposure to the elements, and even how the item is used can cause the electroplated layer to degrade or wear away.
But let’s not be too harsh. Electroplating is far more enduring than paint or other surface treatments. For example:
- Gold-plated jewelry may last several years if cared for properly.
- Chrome-plated car parts can resist corrosion and maintain their shine for decades when not exposed to constant moisture or abrasion.
- Industrial tools with nickel or zinc coatings can extend their working life significantly by resisting rust and wear.
So while it isn’t permanent in the immortal sense, it’s plenty durable if you treat it well.
Factors That Affect the Durability of Electroplating
Electroplating’s lifespan is like a recipe—it depends on the right ingredients and conditions. Here are the main factors that influence how long electroplating lasts:
- Type of Metal Used
Different plating metals have different durability levels. For example:- Gold plating is soft and prone to scratching but doesn’t tarnish.
- Nickel plating is tough and corrosion-resistant, making it great for industrial uses.
- Chrome plating offers a hard, shiny finish but can crack under stress.
- Plating Thickness
Here’s a simple rule: thicker plating = longer life. A thin layer of gold might wear off faster on a ring that’s constantly rubbed against surfaces, whereas a thick layer of zinc on a steel part can endure heavy-duty industrial use. - Environmental Exposure
Moisture, salt, and chemicals are the sworn enemies of electroplating. For example, chrome-plated car parts exposed to salty winter roads can corrode faster than those in dry climates. - Quality of the Plating Process
Cheap electroplating jobs are often the first to fail. High-quality electroplating done by experienced professionals lasts longer because of better cleaning, uniform plating thickness, and precise current control. - Usage and Wear
The more an item is handled or exposed to friction, the faster the plating wears off. For instance, a gold-plated watch clasp will lose its shine far more quickly than a decorative vase.
How Long Does Electroplating Last?
The exact lifespan of electroplating depends on its application and the factors mentioned above. But to give you an idea:
| Item/Use | Expected Lifespan |
|---|---|
| Gold-plated jewelry | 1–5 years (with regular wear) |
| Chrome-plated car parts | 10–20 years (with maintenance) |
| Nickel-plated tools | 10–15 years (with heavy use) |
| Zinc-plated industrial parts | 5–10 years (corrosion resistance) |
Fun tidbit: High-end manufacturers often use thicker plating to extend lifespan, which is why luxury items seem to “age” better.
Real-World Example: Electroplating Longevity
Let’s talk about watches. Take a gold-plated watch, for instance. If worn daily, the plating on the back of the watch (where it contacts the skin) might wear off within a couple of years due to sweat and friction. However, a well-maintained, thicker-plated watch that’s worn occasionally might retain its finish for a decade or more.
Similarly, industrial tools coated with nickel plating can last years in harsh environments, resisting rust and wear—saving companies thousands of dollars in replacements.
While electroplating isn’t permanent in the sense of lasting forever, it is remarkably durable and can last decades with proper care and under ideal conditions. Factors like the type of metal, plating thickness, and environmental exposure all play a role in its longevity. The better the plating process and the gentler the usage, the longer your electroplated items will shine!
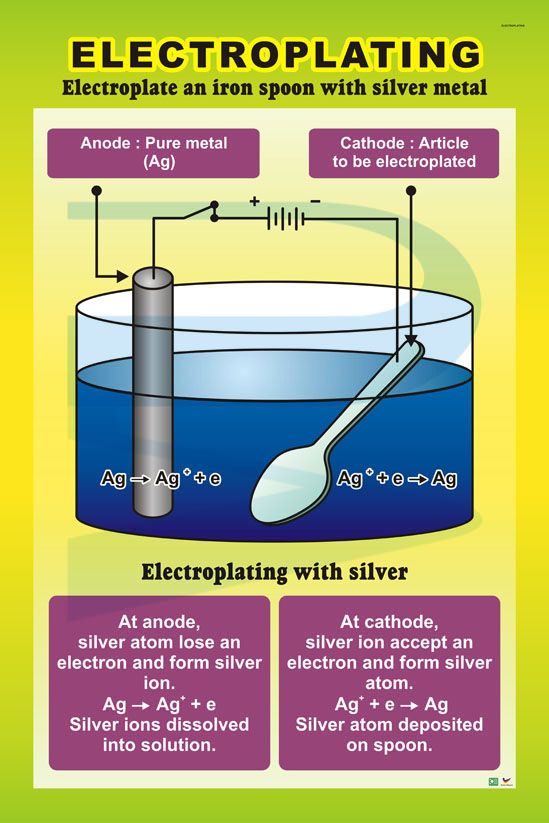
What Causes Electroplating to Wear Off?
Electroplating may seem like a durable solution for protecting and enhancing surfaces, but it isn’t invincible. Over time, various factors can cause the electroplated layer to wear away, dull, or even peel off entirely. Understanding these culprits can help you prevent unnecessary damage and make your electroplated items last longer.
Physical Wear and Abrasion
Think about how often you touch, move, or use an electroplated object. Every action that involves friction contributes to the gradual removal of the plated layer.
- Jewelry is an excellent example: gold-plated rings or bracelets constantly rub against your skin, clothes, or other surfaces. Over time, this contact wears down the delicate plating.
- Industrial tools face even tougher conditions, such as grinding, scraping, or heavy-duty handling, which accelerates the wearing process.
The more an item is exposed to movement or physical force, the faster the electroplating will deteriorate. It’s like how your favorite pair of shoes eventually lose their sole—constant use means inevitable wear.
How to Minimize Physical Wear
- Handle electroplated items gently whenever possible.
- Avoid wearing plated jewelry during activities like exercising, gardening, or cleaning.
- For industrial uses, select metals like nickel or chrome, which offer better resistance to physical wear.
Chemical Reactions and Corrosion
Electroplated surfaces aren’t immune to chemistry. Exposure to certain chemicals, moisture, and even air can kick off reactions that weaken or damage the plating. For example:
- Rust and oxidation: Metals like zinc and nickel are often plated to protect against rust, but if the plating becomes thin or damaged, the underlying material can corrode.
- Acidic or alkaline exposure: Cleaning products or environmental factors like saltwater can cause chemical reactions that degrade the plating. Think about chrome bumpers on cars in coastal areas—they’re prone to pitting and discoloration due to salt-laden air.
Some metals, like gold, are more resistant to corrosion because they don’t react easily with other substances. That’s one reason gold is a popular choice for plating jewelry and electronics.
How to Prevent Corrosion
- Store plated items in dry environments to limit exposure to moisture.
- Avoid harsh cleaning products, opting for mild, non-abrasive solutions instead.
- Consider applying protective coatings like lacquer to seal the plated layer from external elements.
Poor Maintenance or Improper Use
Sometimes, the enemy of electroplating isn’t wear or chemistry—it’s us. Improper care or cleaning can damage the plated layer faster than you’d think. For example:
- Using abrasive materials like rough sponges or steel wool can scratch and strip the plating.
- Over-polishing: While you want your electroplated items to shine, excessive polishing can thin out the metal layer.
- Improper storage: Plated items that are jumbled together in a drawer or exposed to high humidity are more likely to get scratched or tarnished.
Even good intentions—like trying to clean an item more frequently—can backfire if you’re not using the right methods.
Best Practices for Maintenance
- Clean electroplated items with a soft cloth and a gentle cleaning solution.
- Store items individually to prevent them from scratching one another.
- Use polishing sparingly and only when necessary.
Other Factors: Temperature and Stress
Did you know that extreme temperatures can also take a toll on electroplated items? High heat can cause certain plated metals to expand or crack, while sudden temperature changes might create stress that weakens the bond between the plating and the base material. This is particularly relevant for industrial tools or equipment exposed to harsh environments.
How to Protect Against Temperature Damage
- Avoid exposing plated items to extreme heat or cold whenever possible.
- For items used in high-temperature environments, choose metals like chrome or nickel, which are more resistant to heat.
Case Study: Chrome Plating on Cars
Chrome plating is widely used in the automotive industry for its durability and shiny aesthetic. However, one of the biggest challenges is the effect of road salt during winter. Salt accelerates corrosion, leading to pitted or flaking chrome surfaces over time.
In many cases, applying a wax coating or regularly washing the car can help mitigate this issue, preserving the chrome’s luster for years longer. It’s a simple maintenance step that can save you from costly repairs or replacements.
From physical wear to chemical reactions, there are plenty of reasons why electroplating can wear off. However, most of these factors can be managed with proper care, regular maintenance, and a bit of common sense. Think of electroplating like a well-loved piece of clothing—if you treat it with care, it’ll stick around much longer than if you ignore it or subject it to unnecessary roughness.
How to Make Electroplating Last Longer
While electroplating isn’t permanent, there’s a lot you can do to extend its life and keep it looking fresh for years. Whether it’s a cherished piece of jewelry, a decorative home fixture, or industrial equipment, proper care and maintenance can work wonders. Let’s explore the best ways to protect and prolong electroplated surfaces.
Best Practices for Maintenance
A little TLC goes a long way when it comes to electroplating. Here are some easy maintenance tips to prevent unnecessary wear:
- Gentle Cleaning:
Avoid abrasive cleaners or scouring pads that can scratch the plating. Instead, use mild soap and warm water with a soft cloth to gently clean the surface. For stubborn grime, try a soft-bristle toothbrush. - Dry Thoroughly:
After cleaning, make sure to dry the item completely with a soft, lint-free cloth. Water left on electroplated surfaces can lead to spotting or even corrosion over time. - Polish Sparingly:
While polishing can restore shine, overdoing it can thin the plating. Use non-abrasive polishing cloths designed for plated items, and polish only when the surface starts to lose its luster. - Storage Tips:
Store small plated items, like jewelry, in soft fabric pouches or lined jewelry boxes to prevent scratches. For larger items, like plated tableware, use separators (like cloth napkins) to avoid them rubbing against each other.
Applying Protective Coatings
Think of a protective coating as adding a layer of armor to your electroplated item. This additional step can dramatically improve its longevity, especially for items exposed to rough conditions.
- Clear Lacquer or Varnish:
Applying a thin, clear coating can seal the plated surface, protecting it from moisture, air, and physical wear. This is especially useful for decorative items like picture frames or vases. - Wax Coatings:
For automotive parts, applying a coat of wax can help shield chrome-plated surfaces from salt, dirt, and road debris. Plus, it gives the surface a nice shine! - Specialized Plating Sealants:
There are commercial products designed specifically for plated surfaces. These can be a great option if you’re looking for an easy, long-lasting solution.
Replating Services: When and Why?
Sometimes, despite your best efforts, the plating wears off—especially on items that see daily use, like rings, faucets, or industrial tools. Instead of tossing them aside, consider replating.
- What is Replating?
Replating involves removing any remnants of the old layer and applying a new layer of metal. It’s essentially a refresh for your item, restoring its original shine and protective properties. - When Should You Replate?
- If the plated layer has completely worn away in high-contact areas.
- If the item looks uneven or discolored.
- For sentimental items like family heirlooms, to preserve their value.
- How Much Does Replating Cost?
Costs can vary depending on the item’s size and the type of metal used. For example:- Gold replating for jewelry might range from $20–$100, depending on the piece.
- Replating industrial parts or automotive components can cost hundreds of dollars, but it’s often more economical than replacing the entire part.
Pro Tips for Longevity
Here are a few expert hacks to keep electroplated items in tip-top shape:
- Rotate Usage:
For frequently used items, like jewelry, rotate pieces to minimize wear on any single one. This helps reduce the rate of plating loss. - Avoid Water Damage:
Keep plated items away from prolonged exposure to water, especially chlorinated or saltwater, as these can cause tarnishing or corrosion. - Temperature Awareness:
Avoid subjecting plated surfaces to extreme heat or cold, which can cause cracking or warping in the plating layer.
Real-World Example: Protecting Plated Jewelry
Let’s say you have a favorite gold-plated bracelet that’s starting to lose its shine. By following these tips—cleaning it with a gentle cloth, storing it in a soft pouch, and avoiding activities like swimming while wearing it—you could easily extend its life by several years. And when it finally does wear down, a simple replating service can make it look as good as new.
While electroplating isn’t indestructible, proper care, protective coatings, and occasional replating can significantly extend its lifespan. Whether it’s your prized gold-plated earrings or the chrome bumper on your car, these strategies ensure your plated items stay functional and beautiful for as long as possible.