Electroplating is a fascinating process that allows us to coat an object with a thin layer of metal, enhancing its appearance, durability, and resistance to corrosion. Whether you’re electroplating jewelry, automotive parts, or even printed circuit boards, one of the most crucial aspects of the process is knowing how many amps are required for electroplating.
Why does this matter? Because amperage plays a pivotal role in the quality of the electroplating result. Using the wrong amperage can lead to poor adhesion, uneven surfaces, or even ruin the plating altogether.

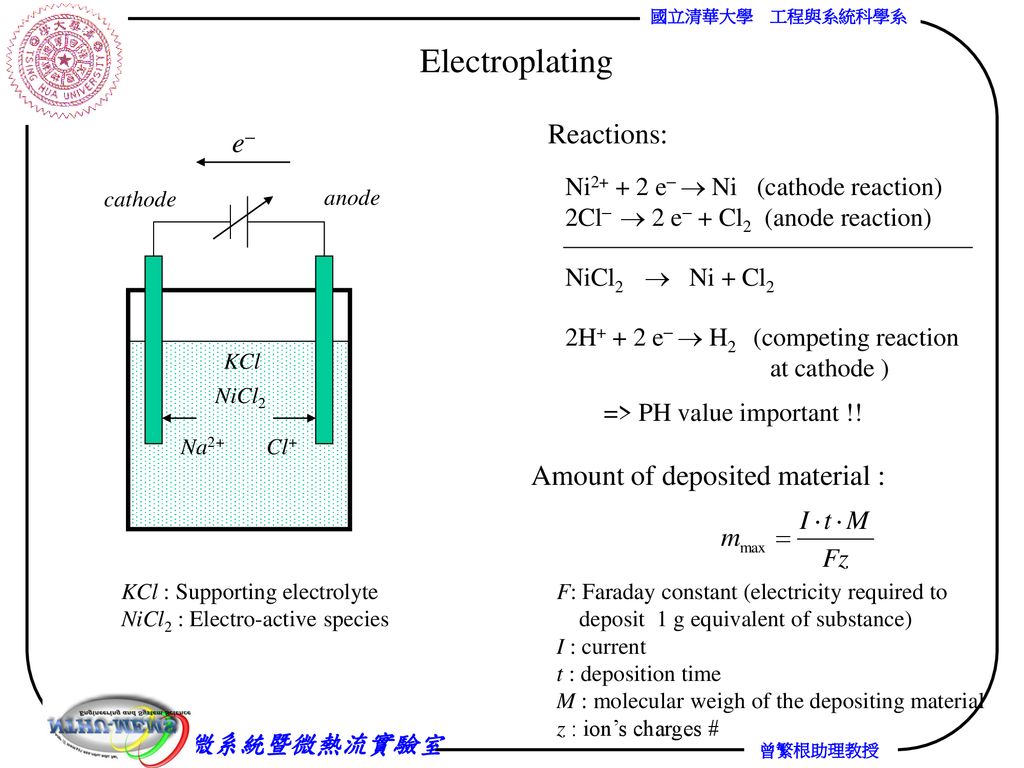
At its core, electroplating is the process of depositing a layer of metal onto an object (known as the substrate or workpiece) using an electric current. This thin metal layer could be made of a variety of metals like gold, silver, copper, nickel, or chrome. The process itself is part chemistry, part engineering, and part artistry.
Here’s how it works:
- Electrolyte Solution: The workpiece (cathode) and a metal anode are submerged in an electrolyte solution. This solution is typically a liquid containing a salt of the metal you want to plate.
- Electric Current: When electricity is passed through the solution, metal ions from the anode are transferred to the cathode (the object being plated). As the metal ions gain electrons, they bond with the workpiece, creating a smooth, metallic coating.
- Plating: Over time, the metal layer builds up on the surface of the workpiece, creating a shiny, durable, and often protective layer. The thickness and quality of this layer depend heavily on several factors, with amperage being one of the most important.
The Role of Amperage in Electroplating
Now that you understand what electroplating is, let’s dive into the importance of amperage. Amperage, or the flow of electrical current, plays a central role in determining the quality and characteristics of the plated surface. It directly influences:
- Plating Speed: Higher amperage generally speeds up the plating process, but it also increases the risk of poor adhesion and uneven plating.
- Coating Thickness: More amps can lead to thicker layers of metal being deposited onto the object.
- Surface Smoothness: Incorrect amperage can result in rough, bumpy, or cracked plating, which is often a sign that the current used was either too high or too low.
Knowing the right amperage is key to getting the desired result. Next we will explain exactly why amperage matters and how using the correct amount can help you avoid common plating issues.
Why is the Amperage Important in Electroplating?
When it comes to electroplating, amperage isn’t something you can just set and forget. It has a significant influence on how your plating turns out, impacting everything from plating speed to finish quality. Let’s take a deeper dive into why amperage is so important.
The Impact of Amperage on Plating Quality
The amount of amps used during electroplating is crucial to achieving a high-quality finish. Here’s how:
- Coating Adhesion: If you use too much amperage, the plating may not bond properly to the surface of the object. The metal could peel or flake off over time, especially if the surface was improperly prepared. On the other hand, if the amperage is too low, the plating might not adhere well enough to withstand wear and tear.
- Surface Smoothness: Higher amperage tends to speed up the plating process, but this can cause uneven deposits, leading to a rough, imperfect surface. The plating might also have a dull or pitted look if the current is too high. Conversely, too little current can result in a rough, porous surface that looks unfinished or unpolished.
- Plating Thickness: The thickness of the plated layer is directly related to the amperage. If you want a thick coating (for protection or aesthetic reasons), you’ll need to use a higher current. However, more amps mean more heat, which can lead to problems with the electrolyte and cause the coating to become brittle or uneven.
- Heat Generation: Higher amperage generates more heat. While a small amount of heat can help the plating process, excessive heat can damage both the electrolyte solution and the workpiece, causing warping or discoloration. For this reason, temperature control is often essential when electroplating with high amperage.
Too Much or Too Little Amperage: The Risks
Using too much current in electroplating can cause several issues:
- Overheating: Excessive current creates too much heat, leading to poor adhesion and potentially damaging the object being plated.
- Rough or Cracked Surface: High amperage can cause the deposited metal to be rough or even crack, which compromises the integrity of the plating.
- Uneven Plating: If the amperage isn’t properly controlled, it can lead to areas of the surface being overplated while others are underplated.
On the flip side, using too little current can also create problems:
- Slow Plating Process: Low amperage results in slow metal deposition, which means you might be waiting hours or even days for the desired coating thickness.
- Weak Layers: Thin coatings produced by low current might not be durable or protective enough for certain applications.
- Inconsistent Coating: Insufficient amperage can lead to blotchy, uneven coatings that fail to achieve the smooth, shiny finish you’re aiming for.
To get the best results, you need to find the sweet spot – the ideal amount of amperage that allows you to achieve a smooth, durable coating at the right speed.
How to Calculate the Required Amps for Electroplating
So, how do you figure out exactly how many amps are required for electroplating? There isn’t a one-size-fits-all answer, as it depends on a variety of factors, including the size of your workpiece, the type of metal you’re using, and the desired plating thickness. Let’s break down these factors and learn how to calculate the right amperage for your project.
What Factors Influence the Amperage for Electroplating?
Several factors determine how much current you need for successful electroplating. Let’s take a look at the most important ones:
- Surface Area of the Object: The larger the surface area of the object being plated, the more current is required. For example, a large automotive part will require more amperage than a small jewelry piece. This is because more surface area needs to be covered with the metal, which requires more energy.
- Thickness of the Plating Layer: If you need a thicker coating, you’ll need to use more amps. A thin layer of plating requires less current, while thicker coatings (e.g., for protection against corrosion or wear) require a higher current.
- Type of Material Being Plated: Different metals have different plating requirements. Gold, silver, and copper all require different amperage levels. Some metals, like chrome, may require higher amperage to achieve a durable, even finish.
- Electrolyte Solution: The composition of the electrolyte solution plays a big role in determining how many amps are needed. For example, plating gold onto a piece of jewelry requires a different electrolyte solution and amperage compared to copper plating on industrial parts.
- Temperature of the Solution: The temperature of your electrolyte solution can affect the plating process. Higher temperatures tend to require lower amperage, while cooler solutions may need more current to maintain the same plating speed.
- Current Density: Current density refers to the amount of electrical current per unit area. In other words, it’s how much current you’re applying to a given surface area of your object. This is typically measured in amps per square foot (A/ft²) and is critical for ensuring uniform plating. Too high a current density can result in overplating or poor adhesion, while too low a density can result in weak or thin layers.
Step-by-Step Guide to Calculating Amps for Electroplating
To calculate how many amps you’ll need, you can use the formula:
Amps = Current Density x Surface Area
For example, if the current density for your plating material is 0.1 amps per square inch (A/in²), and your object’s surface area is 50 square inches, you’d calculate the required amps as follows:
Amps = 0.1 A/in² x 50 in² = 5 amps
This would give you the total current required to plate your object with the desired quality and thickness.
Example Calculation
Let’s say you’re plating a piece of jewelry with silver, and the required current density is 0.2 A/in². If the surface area of the jewelry piece is 12 square inches, the calculation would be:
Amps = 0.2 A/in² x 12 in² = 2.4 amps
So, for this jewelry piece, you’d need approximately 2.4 amps for optimal plating.
Amps vs. Voltage in Electroplating: What’s the Difference?
When diving into electroplating, you’ll quickly come across two terms that are essential for understanding the plating process: amperage (amps) and voltage. While these two are often mentioned together, they serve different functions in the electroplating process. It’s crucial to understand both and how they work together to achieve the perfect electroplated finish.
What’s the Role of Amps in Electroplating?
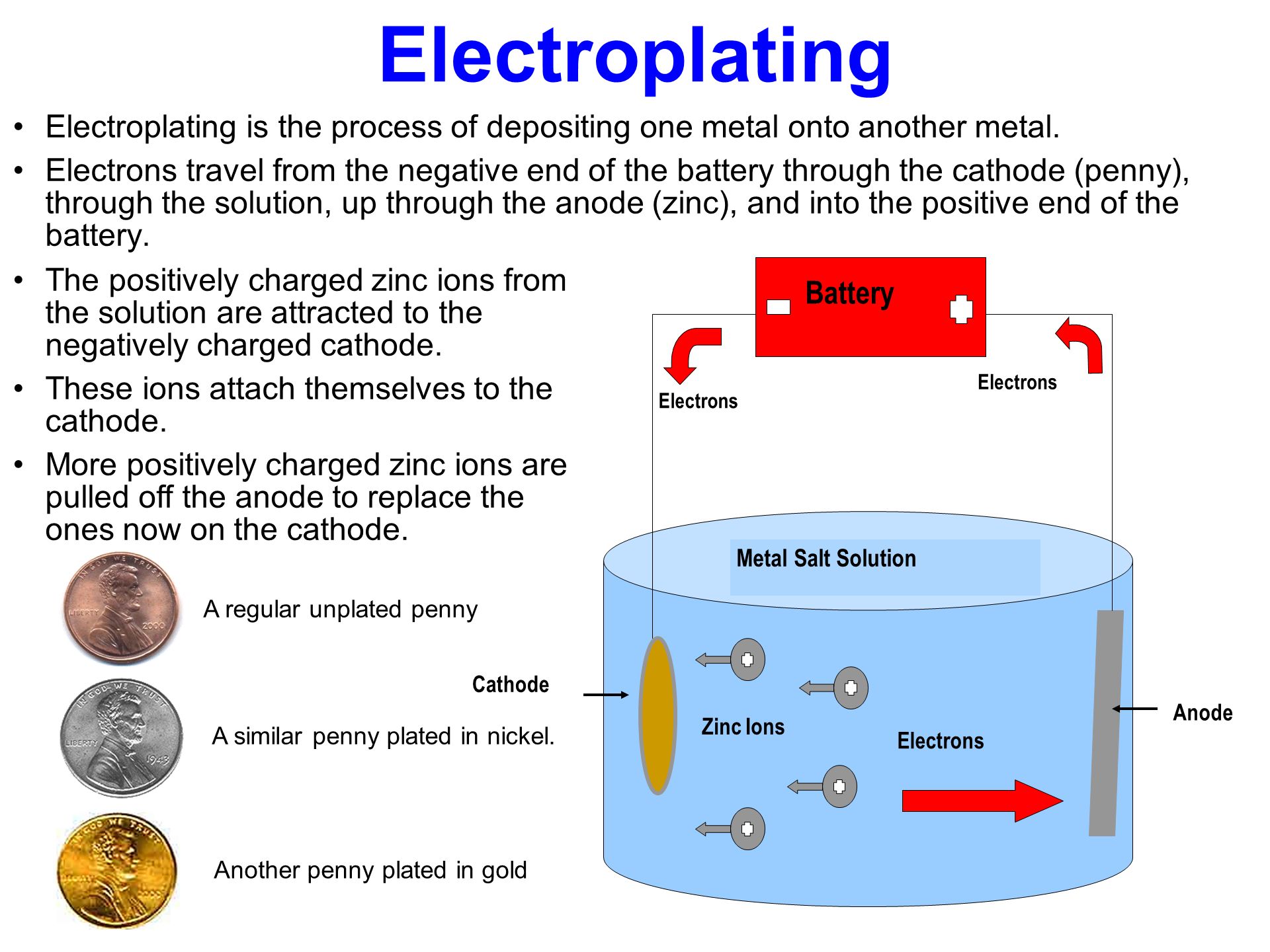
As we’ve already covered, amps represent the amount of electrical current that flows through the electrolyte solution and towards the object being plated. Amps are responsible for the deposition of the metal, meaning they directly affect the thickness and speed of the plating process. If you use the right amount of amps, you’ll achieve a nice, smooth, and durable layer of metal on your workpiece.
Here’s a simple way to think of it:
- Amps are responsible for the volume of metal being deposited onto the object.
- More amps = thicker plating (as long as you maintain proper technique and other conditions).
What’s the Role of Voltage in Electroplating?
Voltage, on the other hand, refers to the electrical potential difference that pushes the current through the electrolyte solution. In simpler terms, voltage is like the “pressure” that drives the current (amps) to flow from the anode to the cathode (your workpiece). While amperage controls the amount of metal being plated, voltage affects how well the process runs.
Voltage, when applied correctly, ensures the current flows steadily and efficiently. Too little voltage won’t push enough current through the solution, resulting in a slow or weak plating process. On the other hand, too much voltage can cause excessive heating, unwanted chemical reactions, or even damage to the workpiece or solution.
The Relationship Between Amps and Voltage
Amps and voltage are closely related but have different impacts. Here’s the key takeaway:
- Voltage sets the current in motion, while amperage controls the amount of metal deposited.
- In electroplating, the amount of voltage you use will determine how many amps flow through the solution. If you apply more voltage, you typically increase the amperage—but this also depends on the resistance of the electrolyte and the setup.
A common misconception is that amperage and voltage are interchangeable. They’re not! They work together to achieve the desired plating results, but you need to balance both.
Adjusting Amperage vs. Voltage in Electroplating
When you’re troubleshooting an issue during electroplating, the decision to adjust amperage or voltage depends on the problem you’re facing:
- For uneven plating or poor adhesion, it’s often best to tweak the amperage (too high or too low). Remember, too much amperage can cause rough, cracked plating, while too little leads to slow, weak deposits.
- For slow or poor metal transfer, adjusting voltage might be necessary. Low voltage means insufficient current, while too high voltage could lead to overheating and poor plating.
In most cases, you’ll want to adjust amperage first, as it’s directly responsible for the plating quality and thickness. But don’t forget that voltage should also be kept in check to maintain a steady and reliable plating process.
How Many Amps Are Needed for Different Electroplating Projects?
The exact amount of amperage required for a given electroplating project varies depending on the size of the object, the metal you’re plating, and the specific requirements for plating thickness and durability. Here, we’ll look at the amperage needed for a few common types of electroplating projects.
Amps for Jewelry Electroplating
Jewelry electroplating is a delicate and precise process. When plating small items like rings, necklaces, or bracelets, the amperage needs to be controlled very carefully to avoid ruining the piece. Typically, the amperage required for jewelry plating is much lower than for larger industrial projects because the surface area is smaller and the plating layers tend to be thinner.
Typical amperage ranges for jewelry electroplating:
- Gold plating: 0.1 – 0.5 amps per square inch (A/in²)
- Silver plating: 0.2 – 0.4 amps per square inch (A/in²)
- Rhodium plating: 0.05 – 0.3 amps per square inch (A/in²)
For example, if you’re plating a ring with silver and the surface area of the ring is 10 square inches, you’d need around 2 amps (0.2 A/in² x 10 in²).
Amps for Industrial Electroplating
Industrial electroplating involves larger objects, such as automotive parts, machinery, or tools. These parts often require thicker plating layers to withstand wear and tear, corrosion, or harsh environments. Consequently, industrial electroplating projects demand much higher amperage compared to smaller, more delicate items like jewelry.
Typical amperage ranges for industrial electroplating:
- Chrome plating: 2 – 5 amps per square foot (A/ft²)
- Nickel plating: 1 – 3 amps per square foot (A/ft²)
- Copper plating: 0.5 – 1.5 amps per square foot (A/ft²)
For instance, if you’re plating a large automotive part with chrome and the part’s surface area is 200 square inches (1.39 square feet), you might need between 2.8 to 6.9 amps for even, high-quality plating.
Amps for PCB (Printed Circuit Board) Electroplating
PCB electroplating is a more specialized process where precision is paramount. Since the surface areas are typically quite small, the amperage required for PCB electroplating is relatively low compared to industrial plating. The goal here is to ensure uniform plating on the tiny circuit pathways and avoid excess metal buildup.
Typical amperage ranges for PCB electroplating:
- Copper plating for PCBs: 0.1 – 0.3 amps per square inch (A/in²)
Given that a PCB usually has a surface area of around 10 square inches, you’d need 1 to 3 amps to plate it evenly with copper. However, PCB plating requires very fine control to avoid damaging the delicate traces on the board.

Common Issues with Amperage During Electroplating
While electroplating can seem straightforward once you understand the basics, a misstep in amperage can lead to a host of problems that compromise the quality of your plating. Let’s explore some of the most common issues caused by incorrect amperage and how to fix them.
What Happens If You Use Too Much Amperage?
Cranking up the amps may seem like a good way to speed up the plating process, but going too high can quickly backfire. Here’s what can happen when the current is excessive:
- Overheating: High amperage increases the heat in the electrolyte solution. This can lead to:
- Decomposition of the electrolyte: Certain solutions break down at high temperatures, releasing gases or byproducts that interfere with the plating process.
- Damage to the substrate: Heat can warp, discolor, or otherwise harm the object you’re trying to plate.
- Poor Adhesion: When the current is too high, the metal ions deposit too quickly. This results in a loose or poorly bonded layer that can flake or peel over time.
- Rough or Cracked Surface: Excessive amperage causes uneven deposition of metal. The plating layer might look bumpy, cracked, or pitted rather than smooth and uniform. This is particularly problematic for parts that need a flawless finish, such as jewelry or automotive components.
- Burning or Dark Spots: High amps can cause localized overheating on the object’s surface, leading to burnt or discolored patches in the plating.
What Happens If You Use Too Little Amperage?
If high amperage creates problems, wouldn’t low amperage be the safer choice? Unfortunately, not. Insufficient current comes with its own set of challenges:
- Slow Plating Process: With low amperage, metal ions move slowly from the anode to the cathode, causing the plating process to drag on. While this might not sound like a big deal, it can be impractical for large or time-sensitive projects.
- Weak or Thin Layers: Low amps often result in a very thin, weak plating layer that offers minimal protection against corrosion or wear. This is especially problematic for industrial parts that need a durable finish.
- Blotchy or Inconsistent Coating: A low current might fail to deposit metal evenly across the surface of the object. This can result in blotchy, patchy finishes that look unprofessional and reduce the functional quality of the item.
- Incomplete Coverage: In extreme cases, insufficient amperage might not deposit enough metal to fully cover the object, leaving exposed areas vulnerable to damage.
How to Troubleshoot Amperage Problems
If you’re experiencing issues during electroplating, the amperage is one of the first things you should check. Here’s how to diagnose and fix common problems:
1. Check Your Formula
- Use the Amps = Current Density x Surface Area formula to confirm that your amperage is in the right range for the size of the object and the metal being plated.
- Double-check the current density recommendations for your specific plating solution and metal.
2. Inspect Your Setup
- Electrolyte Solution: Ensure the solution concentration and temperature are within the recommended ranges. An improperly prepared bath can affect how the current flows.
- Anode and Cathode Placement: Make sure the anode and cathode are positioned correctly and that the anode completely surrounds the workpiece if needed for uniform plating.
3. Adjust the Power Supply
- Gradually increase or decrease the amps while monitoring the results. If you’re unsure, start with lower amperage and slowly raise it until you see consistent, smooth plating.
4. Use an Amp Meter
- A reliable amp meter will help you monitor the current flow in real time. This is especially useful for troubleshooting fluctuating or inconsistent amperage.
5. Conduct Test Runs
- Before working on expensive or critical parts, try plating a test piece with similar material and surface area. This allows you to fine-tune the amperage and identify potential issues before committing to the main project.
6. Balance Your Bath
- Over time, metal ions in the electrolyte solution can become depleted, reducing the plating efficiency. Make sure to replenish or replace the solution as needed.
Case Study: Fixing Amperage Problems in Jewelry Plating
Let’s say you’re plating a small silver bracelet, and you’ve noticed that the surface has developed rough spots and some areas are peeling. After reviewing your process, you realize you’ve been using 3 amps for a surface area that only requires 1.5 amps.
Solution:
- Reduce the amperage to 1.5 amps, which is appropriate for the bracelet’s surface area.
- Allow the electrolyte bath to cool if it’s become too warm due to the excessive current.
- Plate a test piece at the corrected amperage to ensure smooth, even deposition before re-plating the bracelet.
This simple adjustment not only saves the bracelet but also improves your plating results going forward.f