Electroplating is a fascinating process. It’s how we get shiny gold on jewelry, or the sleek silver coating on electronics. But behind the glimmering results lies a highly scientific process where the conditions of the bath, especially the pH, play a crucial role. Understanding how pH affects electroplating can significantly improve the quality and efficiency of your plating work. Whether you’re in jewelry making, industrial coatings, or just a hobbyist trying to plate some metal at home, knowing how to control pH in electroplating can make or break your project.
So, how does pH affect electroplating, you might wonder? In simple terms, pH is a measure of how acidic or basic (alkaline) your solution is. The pH level in your electroplating bath directly influences several factors, from the quality of the coating to the rate of metal deposition. Too high or too low a pH can cause problems, resulting in poor adhesion, uneven plating, or even ruining your entire batch.

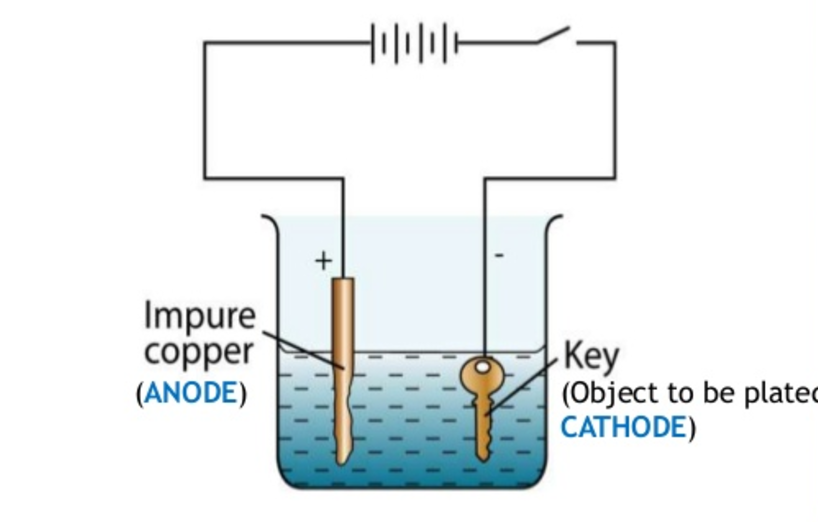
Electroplating is a process that uses electrical current to deposit a thin layer of metal onto the surface of an object. The object that needs plating is made the cathode (negative electrode) in an electrolytic cell, while the metal to be plated is usually made the anode (positive electrode). Between the two electrodes is an electrolyte solution, which contains metal ions that are reduced and deposited onto the cathode when electrical current passes through the bath.
In the electrolyte solution, metal ions are dissolved, and when an electric current is applied, they migrate toward the cathode, where they are reduced and plate the object. The process can be used for various purposes: enhancing appearance, improving corrosion resistance, or even adding a layer of metal to improve conductivity. Common metals used in electroplating include gold, silver, copper, nickel, and chrome.
But here’s the catch: pH is a hidden player in the whole process. It dictates how the metal ions interact with the object being plated and with each other in the solution.
Why is pH Important in Electroplating?
Now that we’ve gotten the basics of electroplating down, let’s dive into why pH is so critical. The pH level of your electroplating solution can determine the success or failure of your entire process. Think of it like baking a cake — the right amount of heat and the correct ingredients are essential to making it rise perfectly. Too much heat (or the wrong temperature), and the cake burns; too little, and it remains raw. The same goes for pH in electroplating: if it’s not in the right range, you can end up with poor plating results.
How does pH affect electroplating? pH controls several aspects of the electroplating process, such as:
- Rate of metal deposition – The higher or lower the pH, the faster or slower metal ions will deposit onto the cathode.
- Quality of the metal coating – pH can affect the smoothness, adhesion, and durability of the plated layer.
- Electrolyte stability – A stable pH ensures that the electrolyte solution remains effective for longer.
Effects of Low pH on Electroplating
Let’s talk about what happens when the pH of your electroplating bath dips too low (more acidic).
- Corrosion of Electrodes: In acidic conditions, the anode (positive electrode) can begin to corrode more quickly, leading to the loss of metal from the anode. This can mess up the overall efficiency of the plating process because you’ll have to replace the anode more often.
- Poor Metal Deposition: Low pH can result in uneven or rough plating. This happens because the plating process tends to favor the deposition of impurities or unwanted elements in the bath. The metal ions might be deposited too quickly, which can result in a flaky, less durable finish.
- Formation of Unwanted By-products: In some cases, if the pH is too low, undesirable chemical reactions might occur, creating hydrogen gas or other by-products. These by-products can interfere with the plating, causing bubbles on the surface or affecting adhesion.
Effects of High pH on Electroplating
Now, let’s see what happens if the pH goes the other direction and becomes too high (more basic or alkaline).
- Hydrogen Evolution: When pH is too high, there’s a tendency for hydrogen gas to evolve at the cathode. This can result in bubbles forming on the surface of the object being plated, which disturbs the plating process and leads to a rough, uneven finish.
- Poor Plating Efficiency: A high pH can slow down the rate of deposition. The metal ions become less mobile, making it harder for them to be reduced onto the cathode. This could result in longer plating times or the need for higher current densities, which can lead to energy inefficiencies.
- Changes in Bath Composition: In extreme cases, a very high pH can cause the electrolyte solution to break down or form precipitates, which can lead to contamination of the bath or clogging of equipment.
:max_bytes(150000):strip_icc()/how-to-define-anode-and-cathode-606452_FINAL-0fb3b21b79564acd9ef7c729a2bcd98e.png)
pH Control in Electroplating Baths
Managing pH in an electroplating bath is a bit like keeping a tightrope walker balanced: it requires precision, regular monitoring, and small adjustments to avoid falling into extremes.
Common Electroplating Baths and Their pH Requirements
Different metals have different “sweet spots” when it comes to pH. These ideal ranges are determined by the chemistry of the metal ions and the type of electrolyte used. Here’s a quick reference guide for the pH requirements of some common electroplating solutions:
| Metal | Typical pH Range | Notes |
|---|---|---|
| Copper (acidic bath) | 1.0–2.5 | Common for decorative and electrical applications. |
| Copper (alkaline bath) | 8.0–10.0 | Often used for functional coatings, like corrosion-resistant layers. |
| Nickel | 3.5–5.5 | Ideal for smooth, bright finishes and industrial applications. |
| Gold | 3.5–6.0 | Gold requires a stable pH for even deposition, particularly in electronics. |
| Silver | 7.5–10.0 | A neutral to slightly basic environment ensures bright, reflective finishes. |
| Zinc | 4.5–5.5 | Zinc plating baths are often slightly acidic for better corrosion resistance. |
Each metal’s pH range is carefully optimized to ensure proper ion reduction, smooth plating, and minimal side reactions. Operating outside these ranges can result in defects, contamination, or even damage to the plating bath itself.
How to Measure and Adjust pH in Electroplating
Maintaining the correct pH requires constant vigilance and the right tools. Here’s a step-by-step guide to keeping your plating bath in balance:
- Measuring pH Levels
- Use a pH meter for the most accurate readings. Modern digital pH meters are easy to use, provide quick results, and are ideal for monitoring the relatively narrow pH ranges required in electroplating.
- For quick checks, pH test strips can be used, but they lack the precision needed for tighter tolerances.
- Regularly calibrate your pH meter using standard buffer solutions (e.g., pH 4, 7, or 10) to ensure accuracy.
- Adjusting pH Levels
- If the pH is too low (acidic):
- Add small amounts of a weak base, such as sodium hydroxide (NaOH) or ammonia (NH₃). Avoid overcorrection by adding the base slowly, allowing time for the bath to stabilize before taking another reading.
- If the pH is too high (basic):
- Introduce an acid, such as sulfuric acid (H₂SO₄) or hydrochloric acid (HCl). Always add acid to water (not the other way around!) to prevent dangerous reactions.
- Pro Tip: Add pH-adjusting chemicals in small increments and stir the solution thoroughly to ensure even distribution. Never add large amounts at once, as this can cause sudden, extreme pH shifts.
- If the pH is too low (acidic):
- Frequency of Checks
- Check the pH at least once per shift for industrial processes or every few hours for smaller-scale operations. This frequency can vary depending on how stable your plating bath typically is.
Best Practices for Maintaining Optimal pH
Keeping the pH in the desired range isn’t just about reacting to changes—it’s about being proactive. Here are some best practices for long-term stability:
- Monitor Bath Composition Regularly: Over time, contaminants and by-products can build up in the bath, affecting pH. Use filtration and periodic replacement of the solution to keep the bath clean.
- Control Temperature: Temperature and pH are closely linked. A sudden temperature spike can alter the chemical balance of the bath, shifting the pH. Use heaters or coolers to maintain a consistent temperature.
- Avoid Overloading the Bath: Plating too many objects at once can disrupt the pH balance. Stick to the recommended load capacity for your plating bath.
- Use Buffers: Adding a buffering agent can help stabilize pH and prevent drastic swings. Common buffers for electroplating include boric acid (for nickel baths) or acetate solutions (for gold baths).
A Real-World Example: The Danger of Ignoring pH
Let’s consider a case study to illustrate how pH control impacts electroplating:
- Scenario: A small-scale manufacturer producing nickel-plated parts ignored regular pH monitoring. Over time, the pH of their bath drifted down to 3.0 (below the optimal range of 3.5–5.5). The result? The nickel plating became uneven, with some parts having dull finishes and others developing pits. When they tried to correct the issue by simply adding more nickel salt to the solution, the plating quality didn’t improve. Why? The root cause—low pH—wasn’t addressed.
- Resolution: After adjusting the pH back to 4.5 using sodium hydroxide and installing a system for regular monitoring, the manufacturer was able to restore plating quality and reduce waste.
Mastering pH control is essential for achieving high-quality electroplating results. But what happens when things go wrong?
Troubleshooting pH Issues in Electroplating
As with any intricate process, things don’t always go according to plan. Even when you carefully monitor pH levels, unforeseen issues can still arise, and understanding how to troubleshoot pH-related problems in electroplating is key to keeping your process running smoothly.
Common pH-Related Problems in Electroplating
- Poor Adhesion or Peeling of Plating
- Symptoms: After plating, the metal coating doesn’t bond well with the substrate, causing it to peel or flake off easily. This is often due to irregular or poor metal deposition.
- Possible Cause: The pH of the bath may be outside of the optimal range. When the pH is too low or too high, it can disrupt the plating process, leading to poor adhesion. At low pH, the metal may deposit too quickly and unevenly, while at high pH, the surface may not be able to receive the metal ions properly.
- Solution: Adjust the pH to the ideal level for the metal you’re plating. For example, nickel plating usually requires a pH between 3.5 and 5.5, while copper plating should be around 1.0 to 2.5 in an acidic bath. Check the bath regularly and adjust the pH as needed.
- Dull or Uneven Finishes
- Symptoms: The plating doesn’t have the expected glossy, smooth finish. Instead, it’s dull, uneven, or rough to the touch.
- Possible Cause: This is often a sign of an unstable pH or improper current density. If the pH is too low, metal ions may deposit irregularly, causing the surface to appear rough or patchy. Alternatively, if the pH is too high, hydrogen gas can evolve at the cathode, causing bubbles that disturb the plating surface.
- Solution: Ensure that the pH is within the ideal range for your plating solution. Additionally, consider adjusting the current density or temperature to help achieve a smoother finish.
- Inconsistent Thickness of Plating
- Symptoms: Some areas of the plated object have a thicker coating than others, leading to an uneven surface.
- Possible Cause: The pH may be fluctuating during the plating process. If the pH is too high or low, the plating rate can become erratic, causing the plating to deposit unevenly.
- Solution: Stabilize the pH at the recommended level for the metal you are plating. Regularly monitor and adjust the pH to avoid fluctuations. Using a buffering agent can also help to maintain pH stability throughout the plating process.
- Hydrogen Gas Evolution (Bubbling)
- Symptoms: You notice bubbling or the evolution of gas, particularly at the cathode, which results in poor surface quality and low plating efficiency.
- Possible Cause: This issue is usually caused by a high pH (alkaline conditions) in the bath. When the pH is too high, hydrogen ions (H⁺) are less likely to be available to reduce metal ions at the cathode, and instead, hydrogen gas (H₂) is released, leading to bubbling.
- Solution: Adjust the pH of the solution back to a slightly acidic or neutral level, depending on the metal being plated. Avoid letting the pH get too high, as this can disrupt the plating process.
Solutions for pH-Related Problems
- Correcting pH Imbalance
- The most obvious solution to any pH-related issues is to adjust the pH back to its optimal range. If the pH is too acidic, add a mild base like sodium hydroxide or ammonium hydroxide. If it’s too alkaline, carefully add an acid such as sulfuric acid or hydrochloric acid. Always add chemicals slowly and monitor the pH as you go to avoid overshooting the target.
- Consistent Monitoring
- One of the best ways to avoid pH-related problems is regular monitoring. Invest in a high-quality digital pH meter or an automated pH control system. These tools can help you keep the bath in optimal range throughout the plating process without needing constant manual checks.
- Control the Temperature
- Remember that temperature and pH go hand in hand. A rise in temperature can cause the pH to fluctuate, and the solution might become more acidic or basic. Keep the temperature of the plating bath stable and within the recommended range for your metal and solution. This will help keep both the pH and the plating process consistent.
- Use Buffers for Stability
- If you find that your bath is particularly sensitive to pH changes, consider using buffer solutions. Buffers are chemical mixtures that can help maintain a stable pH even when acids or bases are added. They’re an excellent way to prevent pH from swinging too drastically, which can lead to inconsistent plating.
A Real-World Example: Solving pH-Related Plating Issues
Consider a scenario with an industrial company that plates nickel onto steel parts for automotive applications. The company noticed that the finished parts were suffering from poor adhesion and uneven thickness, even though the bath composition was correct. After investigating, it turned out that the pH of the bath was drifting too low over time, causing poor metal deposition and rough surfaces.
The solution was to adjust the pH to 4.5–5.0 (the ideal range for nickel plating) and to implement a routine check on the pH levels every four hours. With these adjustments, the company was able to significantly improve the adhesion, finish quality, and plating uniformity.