When it comes to manufacturing and finishing materials, electroplating is a popular technique. It’s often used in industries ranging from electronics to jewelry, automotive, and beyond. But the big question on many minds is: does electroplating add thickness to the material being plated? And if so, how much?
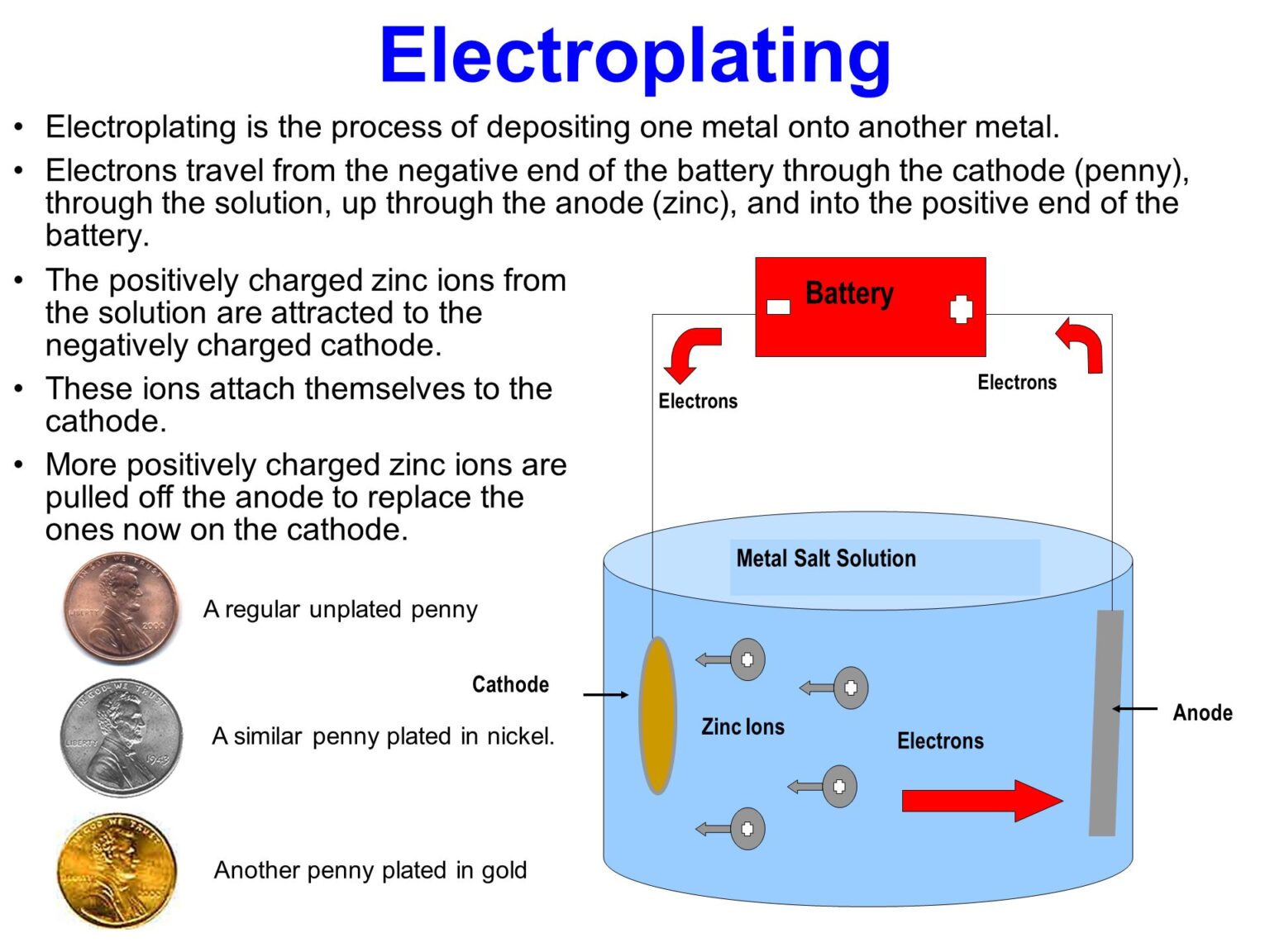
Before we answer the pressing question about thickness, it’s important to understand what electroplating is. In simple terms, electroplating is a process in which a thin layer of metal is deposited onto the surface of an object using electrical current. This happens when the object is placed into a solution containing metal salts, and when current is passed through it, metal ions are reduced and form a coating on the object’s surface.
But why would anyone want to do that? Well, electroplating can serve many purposes, including:
- Improving the appearance of an object (for example, gold-plating jewelry or silver-plating cutlery).
- Enhancing corrosion resistance to prevent rusting and wear (common in automotive or electronic parts).
- Increasing the durability of an item, especially when it’s subject to friction or heavy use (think of the chrome-plated parts in cars).
- Improving electrical conductivity for components in electronics.
So while electroplating doesn’t change the material itself, it certainly alters its properties by adding a coating. But the big question remains: Does it add thickness to the material? Let’s explore that next!
How Does Electroplating Work?
To answer whether electroplating adds thickness, we first need to break down how the process works and understand what happens during the plating process. Here’s how it goes:
- Preparing the Surface: The object to be electroplated (called the substrate) is thoroughly cleaned to remove dirt, oils, or other impurities. This step is critical for ensuring the plating adheres properly.
- The Electroplating Bath: The object is submerged into an electrolyte solution containing metal salts. This solution serves as the source of metal ions for the plating process. For example, if you’re plating with gold, the solution would contain gold salts.
- Applying Electrical Current: When the object is connected to a power source, an electric current is passed through the electrolyte solution. The current causes the metal ions to migrate and bond to the object’s surface. This forms a layer of metal over the substrate.
- Layer Formation: Over time, more metal accumulates, creating a coating on the surface. The thickness of this coating depends on several factors we’ll get into shortly.
This process results in a metallic layer that is firmly bonded to the object, but does electroplating actually add thickness? The short answer is: Yes, it does.
But here’s the catch: The thickness added by electroplating is usually quite small, typically in the range of microns (millionths of a meter). The exact thickness can vary depending on the type of plating, the plating time, and the conditions used.
Does Electroplating Add Thickness to the Material?
Now, let’s tackle the heart of the matter: Does electroplating really add thickness to the material? Yes, it does.
When metal is electroplated onto an object, it accumulates in layers on the surface. This means that the object becomes slightly thicker. However, the increase in thickness is usually very small and can vary depending on several factors:
- Plating time: The longer the plating process, the more metal is deposited, and the thicker the coating will be.
- Current density: The higher the current applied, the faster the metal will be deposited. This can increase the thickness of the plating.
- Metal type: Some metals are deposited in thinner layers than others, depending on their characteristics. For example, gold plating typically results in a thinner coating compared to nickel plating.
The thickness of electroplating is usually measured in microns, with some coatings being just a few microns thick. For reference, a human hair is approximately 70 microns thick, so even a “thick” electroplated layer is relatively thin compared to the material it’s covering.
Here’s a quick overview of the typical plating thicknesses for common metals:
| Metal | Typical Plating Thickness (in microns) |
|---|---|
| Gold | 0.1 to 2 microns |
| Nickel | 5 to 25 microns |
| Chrome | 5 to 20 microns |
| Silver | 1 to 5 microns |
As you can see, nickel and chrome plating tend to add more thickness compared to gold or silver plating.
What Factors Affect Electroplating Thickness?
So, if you’re hoping for a thick, durable layer of metal, it’s important to know the factors that impact the thickness of the electroplating. Here are the main things to consider:
- Plating Time:
- The longer you let the object sit in the electroplating bath, the more metal will accumulate on its surface, leading to a thicker coating. It’s like baking a cake – the longer it’s in the oven, the more it rises.
- Current Density:
- Higher current densities lead to faster metal deposition. However, this can also lead to uneven plating, so it’s essential to control the current carefully to avoid undesirable results like “burning” the surface or forming a rough texture.
- Temperature and Bath Composition:
- The temperature of the electrolyte bath and the specific composition of the solution can influence the deposition rate and uniformity of the plating. A hotter bath may speed up plating but could also cause issues like poor adhesion if not carefully managed.
- Base Material:
- Different materials will absorb the plating differently. A more porous or rough surface can allow for thicker layers, while smoother surfaces may result in thinner coatings. The type of metal being plated also impacts how much adheres to the surface.
Electroplating does add thickness to materials, but the thickness added is typically quite thin and varies based on several factors. While the increase in thickness is usually small, it plays a significant role in the material’s durability, appearance, and overall performance.
How Does Electroplating Thickness Affect the Final Product?
Now that we’ve established that electroplating does indeed add thickness to the material, let’s dive into how this thickness impacts the final product. Depending on the thickness of the electroplated layer, the final result can vary dramatically. From enhancing aesthetic appeal to improving functionality, the effect of added thickness can influence everything from durability to appearance. Let’s explore how.
Aesthetic Appearance
The thickness of the electroplated layer directly affects the visual outcome of the product. For example, when you electroplate an object with gold, the thickness of the gold layer can dramatically alter how shiny or reflective the object looks. A thin layer of gold might give a nice, subtle shimmer, while a thicker layer can create a more luxurious and opulent look.
- Thin plating (less than 1 micron): Ideal for a more delicate, subtle appearance. It may be used in items like costume jewelry or decorative elements where a light touch of the metal is desired.
- Thick plating (more than 1 micron): For jewelry or high-end items, thicker plating can give the object a more premium, glossy finish, enhancing its visual appeal.
However, it’s important to note that too thick a plating can sometimes lead to problems. If the coating is too thick, it may cause the surface to become brittle, and in some cases, it can even lead to the coating peeling off over time if the adhesion isn’t strong enough.
Functional Benefits
Beyond appearance, added thickness through electroplating can bring numerous functional advantages to a material. These benefits are often seen in applications where the metal plating serves to enhance the physical properties of the object. Some examples include:
- Corrosion Resistance:
- A thicker electroplated layer can provide better protection against corrosion. For instance, nickel plating on automotive parts helps to prevent rust and weathering.
- Wear Resistance:
- Electroplating can be used to improve a material’s ability to withstand friction and wear. For example, chrome plating is often applied to engine components because it forms a hard, durable surface that resists abrasion.
- Electrical Conductivity:
- In electronic devices, a layer of silver or gold can enhance electrical conductivity, making the product more efficient and reliable.
- Improved Durability:
- Adding a thicker coating can protect delicate base materials (such as plastic or aluminum) from damage due to physical impact, chemicals, or extreme temperatures.
A thicker electroplated layer generally means longer-lasting and more durable products, especially when it comes to functional items that will be exposed to harsh conditions, like tools or automotive parts.
Challenges of Too Much Thickness
However, there is such a thing as too thick when it comes to electroplating. While a thicker layer of metal might seem like an obvious advantage, there are several potential issues to be aware of:
- Increased Weight:
- Thicker coatings can add extra weight to the object. This might not be a major concern for small items, but in the case of large, precision-engineered components (like in aerospace), even a slight increase in weight can have a significant impact.
- Brittleness:
- If the plating is too thick, the metal layer might become brittle and prone to cracking or flaking off. This is particularly problematic with decorative items like jewelry, where you want the plating to be durable but still flexible enough to endure wear and tear.
- Adhesion Issues:
- A thick electroplated layer can sometimes struggle to adhere properly to the base material, leading to delamination or peeling. This is particularly true if the plating process is rushed or the surface wasn’t properly prepped.
The key takeaway here is that electroplating adds thickness, but balance is essential. Too little thickness and you lose out on the functional benefits, but too much thickness and you risk compromising the material’s integrity.
Types of Electroplating and Their Thickness Considerations
Now that we’ve covered the general effects of electroplating thickness, let’s explore how different types of electroplating can influence the final thickness and functionality of a coating. Different metals are used for different purposes, and the amount of thickness added varies depending on the material being used.
Gold Plating
Gold electroplating is one of the most common applications for electroplating, particularly in the jewelry industry. Gold plating offers both aesthetic and functional benefits, but how much thickness is typically added?
- Typical Thickness: Gold plating is usually applied in very thin layers, ranging from 0.1 microns to 2 microns. A thicker gold coating might be used in jewelry that requires a more durable finish, but for most applications, a thin layer is sufficient to achieve the desired aesthetic.
Gold plating is great for adding a touch of luxury to an item, but because the layer is thin, it’s not suitable for high-wear applications. Over time, gold-plated items may show signs of wear and the underlying metal might start to show through.
Nickel Plating
Nickel electroplating is often used for functional purposes—it provides excellent corrosion resistance and durability. Nickel plating is commonly found in the automotive and aerospace industries.
- Typical Thickness: Nickel plating typically adds 5 to 25 microns of thickness. The thickness can vary based on the specific use—automotive parts might need a thicker coating to resist wear and corrosion, while lighter, decorative items might require a thinner layer.
Nickel plating is often applied in situations where the goal is protective functionality rather than aesthetics. The added thickness provides a strong, long-lasting surface that can withstand exposure to harsh conditions.
Chrome Plating
Chrome is another popular metal for electroplating, particularly in the automotive industry, where it’s used for decorative finishes on bumpers, grilles, and wheels. But does chrome plating add significant thickness?
- Typical Thickness: Chrome plating adds a layer that is typically 5 to 20 microns thick. While the thickness of chrome plating might seem modest, it offers several advantages like corrosion resistance and high wear resistance.
Chrome plating is often used when a shiny, reflective surface is desired, but it also provides a functional barrier that extends the lifespan of the object.
Silver Plating
Silver electroplating is often used to enhance the appearance of items while providing a level of corrosion resistance. It’s common in the creation of cutlery, jewelry, and high-end electronics.
- Typical Thickness: Silver plating usually adds 1 to 5 microns of thickness. The plating is typically thin enough to enhance the aesthetic properties of an item while offering some functional benefits, like improved electrical conductivity.
While silver plating is great for decorative purposes, it’s generally not as durable as nickel or chrome plating. Over time, it can tarnish, especially when exposed to air and moisture.
How to Measure Electroplating Thickness?
Once you’ve applied electroplating, it’s essential to measure how thick the deposited layer is. Accurate measurement ensures that the electroplated layer has the right properties and functionality for your specific needs.
Here are some of the most common methods used to measure electroplating thickness:
- Micrometers: Simple tools like micrometers can measure the physical thickness of a plated layer, though they can be a bit tricky to use for very thin layers.
- X-Ray Fluorescence (XRF): This is a non-destructive method that measures the thickness of the plating by analyzing how X-rays interact with the material. XRF is widely used in industries like electronics where precise measurements are crucial.
- Eddy Current Testing: This technique uses electromagnetic fields to detect the thickness of the metal coating. It’s commonly used in industries where the substrate is conductive.

Advantages of Electroplating in Adding Thickness
Now that we’ve explored how electroplating affects the thickness of materials, let’s discuss the advantages of using this process. By adding a layer of metal to a substrate, electroplating offers several benefits—ranging from enhanced durability to improved aesthetics. Here’s a closer look at why so many industries rely on electroplating.
Improved Durability
One of the most significant advantages of electroplating is the added durability it provides. By applying a metal layer, electroplating makes objects more resistant to damage from environmental factors like moisture, heat, and wear. This is particularly useful in industries where the materials need to endure harsh conditions.
For example:
- Automotive Parts: Chrome plating on car bumpers and wheels provides a hard, shiny surface that resists wear, corrosion, and weathering. It not only enhances the car’s appearance but also prolongs the lifespan of the parts.
- Tools and Equipment: Nickel plating on tools and machinery adds thickness and provides excellent protection against rust, scratches, and heavy usage. A thicker plating can ensure that the tools last longer under stress.
The added thickness from electroplating creates a protective shield that extends the product’s life, reducing the need for frequent replacements or repairs.
Cost-Effectiveness
Electroplating is often a more cost-effective method for improving the properties of a material compared to other coating techniques. Rather than using solid, expensive metals or materials, electroplating allows manufacturers to coat cheaper substrates with a thin, but highly effective, layer of metal. This can significantly reduce costs, especially for high-value metals like gold or platinum.
For example, gold-plated electronics provide the conductivity and corrosion resistance of gold without the high cost of using solid gold components. Similarly, silver-plating can be used in decorative items to achieve a shiny, high-end look without the need for solid silver.
In terms of thickness, electroplating allows manufacturers to adjust the coating thickness based on the budget and needs of the project. This flexibility in how thick the coating is can further optimize costs while achieving the desired functional or aesthetic result.
Customization Options
One of the most attractive features of electroplating is the customizability it offers. Since the process allows manufacturers to control the amount of plating and the type of metal used, it’s possible to tailor the thickness and composition of the plating to meet specific needs.
For example:
- Electronics: In the electronics industry, gold plating might be applied with a very thin layer (around 0.1 microns) to provide excellent conductivity while maintaining a lightweight, compact form.
- Jewelry: On the other hand, gold-plated jewelry might use a thicker layer for a more durable, luxurious appearance. In contrast, silver-plating might be applied in a thin layer to keep the cost low while still providing an attractive finish.
This ability to control the thickness of the plating makes it a versatile option for a variety of industries, where specific functionality or aesthetics are required.
Common Myths About Electroplating and Thickness
Electroplating is a well-established process, but there are still some common myths and misunderstandings about how it works, especially regarding the thickness of the coating. Let’s debunk a few of the most prevalent myths.
Myth 1: Electroplating Adds Significant Mass to the Material
One common misconception is that electroplating adds a lot of weight to an object. In reality, the metal layers deposited by electroplating are extremely thin, and even though they add some thickness to the material, they don’t significantly increase its mass.
For example, a 10-gram piece of jewelry might weigh only a fraction of a gram more after electroplating with gold, despite the gold layer being several microns thick.
While plating does indeed increase the material’s thickness, the added mass is usually minimal and doesn’t substantially change the weight of the item unless you are plating with an exceptionally dense metal like gold or platinum.
Myth 2: Electroplating Always Adds Thick Coatings
Another myth is that electroplating always results in thick coatings. This is far from the truth! The thickness of the plating depends entirely on the plating parameters. For example:
- Gold plating can be as thin as 0.1 microns, which would add almost no noticeable thickness.
- Nickel or chrome plating, however, can add coatings up to 25 microns or more, making them significantly thicker.
Electroplating offers flexibility in controlling the plating thickness based on the application’s requirements, so it’s not always about thick layers.
Myth 3: Electroplating Can Only Add Small Thickness
This myth is often perpetuated by people who associate electroplating with decorative finishes. While it’s true that electroplating is often used for thin coatings (like in jewelry), it can actually add considerable thickness when required. In applications like industrial equipment or automotive parts, electroplating with metals like nickel or chrome can result in plating thicknesses that are thick enough to provide protection against corrosion, wear, and harsh conditions.
Is Electroplating the Best Method for Adding Thickness?
Now, you may be wondering if electroplating is the best method for adding thickness to materials. The answer depends on the specific application and what you need from the coating. There are several methods available for adding thickness, and each comes with its own set of advantages and drawbacks.
Alternative Methods for Adding Thickness
While electroplating is highly effective, it’s not the only method for adding thickness to a material. Some alternatives include:
- Cladding: This process involves bonding a layer of metal to a base material, creating a thicker, more durable layer. Cladding is often used for materials that require extreme strength or resistance to high temperatures.
- Coating: Like electroplating, coating adds a layer of material, but it can be done using techniques such as spraying or dipping. Coatings tend to be thicker and can provide better protection in some situations.
- PVD (Physical Vapor Deposition): This technique is used to deposit thin layers of metal onto a surface, similar to electroplating, but without the use of electricity. PVD can add coatings with different thicknesses depending on the application.
When Should You Choose Electroplating?
Electroplating is the preferred method when you need to:
- Improve the material’s aesthetic appearance (like adding a shiny gold layer to jewelry).
- Add a functional layer of protection (for example, corrosion-resistant nickel plating on machinery parts).
- Achieve a precise, customizable layer thickness for specific needs (from thin coatings for electrical conductivity to thick coatings for wear resistance).
If you’re looking for precision, cost-effectiveness, and a variety of metal options, electroplating is often the best choice for adding thickness, especially when you need it in thin, controlled layers.
Does Electroplating Add Thickness?
So, does electroplating add thickness? The answer is a resounding yes! Electroplating adds a layer of metal to a substrate, increasing its thickness, though the amount added is typically quite thin. This added thickness can significantly enhance both the appearance and functionality of the object, whether you’re adding a protective coating to a car part or giving a piece of jewelry a luxurious finish.
The amount of thickness added depends on various factors, including plating time, current density, and the type of metal used. So, while electroplating doesn’t turn a material into a brick, it does provide valuable benefits like improved durability, corrosion resistance, and a sleek finish.

FAQ :
Let’s wrap things up with a quick FAQ to answer some of the most common questions people have about electroplating and its effect on thickness.
How thick can electroplating get?
Electroplating can add a layer of metal ranging from a few microns to several tens of microns thick. For example, gold plating typically ranges from 0.1 to 2 microns, while nickel and chrome plating can be as thick as 25 microns or more. The thickness depends on the application and the desired effect.
In industries where wear resistance or corrosion protection is crucial, thicker layers are typically applied. In decorative applications, thinner layers may be preferred to maintain the lightness and cost-effectiveness of the item.
Does electroplating affect the original material’s properties?
Yes, electroplating can slightly alter the original material’s properties, but it’s usually a beneficial change. The electroplated layer enhances qualities like corrosion resistance, wear resistance, and aesthetic appeal. However, the underlying material remains largely unchanged except for the added metal layer.
For example, a base material like copper might become more resistant to corrosion when coated with nickel, but the base copper’s properties don’t drastically change. In some cases, if the coating is too thick or not applied properly, it can lead to adhesion problems or cause the coating to peel off over time.
What is the difference between electroplating and electroless plating?
Great question! Both electroplating and electroless plating are used to apply metal coatings to a substrate, but there’s a key difference in the process:
- Electroplating requires an electric current to deposit the metal onto the surface. The object to be plated is immersed in an electrolyte solution containing metal salts, and the current facilitates the metal’s deposition onto the surface. Electroplating allows for precise control over the thickness of the layer.
- Electroless plating, on the other hand, does not rely on an electric current. Instead, it uses a chemical reaction to deposit metal onto the surface. This process can be used on materials that are difficult to plate using electroplating, such as non-conductive materials like plastics. Electroless plating can still add thickness, but it tends to produce a more uniform coating compared to electroplating, particularly for complex shapes.
In terms of thickness, electroplating is often better suited for applications requiring precise control over thickness, while electroless plating may be better for uniformity.
Can electroplating be used to repair worn-out parts?
Yes, electroplating is often used in repair and restoration work, especially for parts that have become worn out over time. For example, components such as gears, shafts, and tools can be plated to restore their original dimensions and improve their performance.
The process works by adding metal to the worn areas, effectively building up the surface and filling in the gaps created by wear. Nickel plating is commonly used for this purpose because it provides a hard, durable layer that can withstand friction and wear.
In the case of jewelry repair, electroplating can restore a gold or silver finish, repairing the worn sections by adding a fresh layer of metal, and returning the item to its original appearance.
Is there any way to prevent the plating from becoming too thick?
Yes, there are several ways to control and prevent over-thick plating:
- Control plating time: The longer the material is immersed in the plating bath, the thicker the coating will be. By monitoring the plating time, you can ensure that the plating doesn’t get too thick.
- Adjust current density: Higher current density generally leads to faster deposition, which can increase thickness. Lower current densities can slow down the deposition rate and allow for thinner coatings.
- Use of plating additives: Certain chemicals added to the electrolyte solution can help control the growth of the plating layer. These additives may help produce a smooth, uniform coating without excessive thickness.
- Plating temperature: By carefully controlling the temperature of the plating bath, you can influence the rate of metal deposition and prevent the coating from becoming too thick or uneven.
These methods allow manufacturers to achieve the desired thickness without the potential downsides of an overly thick coating, such as brittleness or excessive weight.
Does Electroplating Add Thickness?
Electroplating does add thickness to the material it is applied to. The increase in thickness is usually quite thin, typically measured in microns, but it provides several advantages depending on the application. Whether you’re looking for improved durability, corrosion resistance, or just a shiny new surface, electroplating is an effective and cost-efficient way to achieve your desired results.
The thickness of the electroplated layer can be precisely controlled, and it can have a significant impact on both the appearance and functionality of the final product. However, it’s important to remember that the process is not about adding bulk—it’s about enhancing the properties of the material while keeping the added thickness minimal and effective.
So, the next time you ask, “Does electroplating add thickness?” you’ll have all the details you need to answer confidently. Electroplating might not turn a piece of material into a thick, heavy chunk, but it can certainly transform it in ways that make it more durable, attractive, and functional.